A distinct metabolic and epigenetic state drives trained immunity in HSC-derived macrophages from autoimmune mice
IF 19.8
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
Abstract
Here, we investigate the contribution of long-term hematopoietic stem cells (HSCsLT) to trained immunity (TI) in the setting of chronic autoimmune disease. Using a mouse model of systemic lupus erythematosus (SLE), we show that bone marrow-derived macrophages (BMDMs) from autoimmune mice exhibit hallmark features of TI, including increased Mycobacterium avium killing and inflammatory cytokine production, which are mechanistically linked to increased glycolytic metabolism. We show that HSCs from autoimmune mice constitute a transplantable, long-term reservoir for macrophages that exhibit the functional properties of TI. However, these BMDMs exhibit reduced glycolytic activity and chromatin accessibility at metabolic genes while retaining elevated expression of TI-associated transcriptional regulators. Hence, HSC exposed to autoimmune inflammation can give rise to macrophages in which the functional and metabolic properties of TI are decoupled. Our data support a model in which TI is characterized by a spectrum of molecular and metabolic states driving augmented immune function.
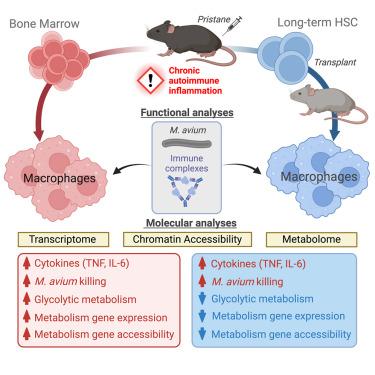
独特的代谢和表观遗传学状态驱动着自身免疫小鼠造血干细胞衍生巨噬细胞的训练有素的免疫力
在此,我们研究了长期造血干细胞(HSCsLT)在慢性自身免疫性疾病中对训练免疫(TI)的贡献。利用系统性红斑狼疮(SLE)小鼠模型,我们发现来自自身免疫性小鼠的骨髓源性巨噬细胞(BMDMs)表现出TI的标志性特征,包括分枝杆菌杀伤力增强和炎性细胞因子产生,而这些特征在机理上与糖代谢增强有关。我们的研究表明,来自自身免疫小鼠的造血干细胞构成了一个可移植的、长期的巨噬细胞储库,这些巨噬细胞具有 TI 的功能特性。然而,这些 BMDMs 表现出糖酵解活性和代谢基因染色质可及性的降低,同时与 TI 相关的转录调控因子的表达仍保持升高。因此,暴露于自身免疫炎症的造血干细胞可产生巨噬细胞,其中 TI 的功能和代谢特性是分离的。我们的数据支持这样一个模型:TI 的特征是一系列分子和代谢状态驱动免疫功能增强。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


