The hippocampal CA2 region discriminates social threat from social safety
IF 20
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
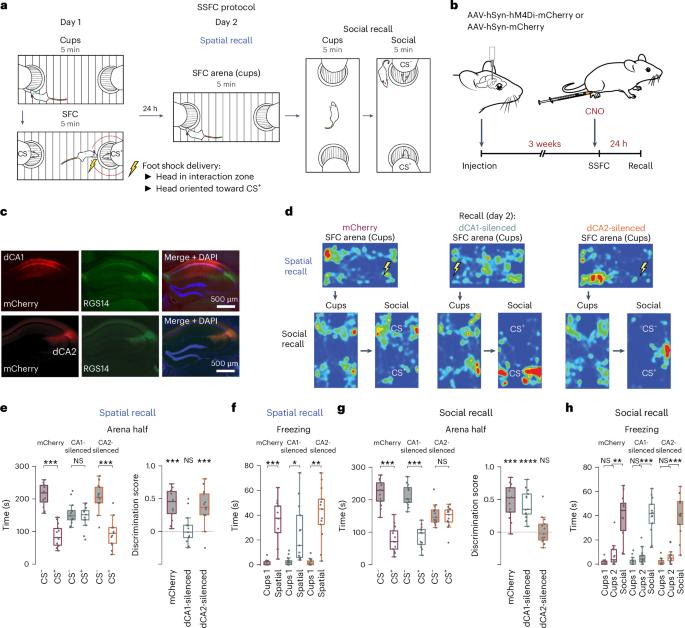
The dorsal cornu ammonis 2 (dCA2) region of the hippocampus enables the discrimination of novel from familiar conspecifics. However, the neural bases for more complex social–spatial episodic memories are unknown. Here we report that the spatial and social contents of an aversive social experience require distinct hippocampal regions. While dorsal CA1 (dCA1) pyramidal neurons mediate the memory of an aversive location, dCA2 pyramidal neurons enable the discrimination of threat-associated (CS+) from safety-associated (CS−) conspecifics in both female and male mice. Silencing dCA2 during encoding or recall trials disrupted social fear discrimination memory, resulting in fear responses toward both the CS+ and CS− mice. Calcium imaging revealed that the aversive experience strengthened and stabilized dCA2 representations of both the CS+ and CS− mice, with the incorporation of an abstract representation of social valence into representations of social identity. Thus, dCA2 contributes to both social novelty detection and the adaptive discrimination of threat-associated from safety-associated individuals during an aversive social episodic experience. The hippocampal dorsal CA2 enables the recognition of novel conspecifics. Kassraian et al. show that it is also required for discriminating safety- versus threat-associated conspecifics and that its disruption gives rise to generalized social avoidance.

海马 CA2 区区分社会威胁和社会安全
海马背侧胼胝体2(dCA2)区域能够辨别新的和熟悉的同种动物。然而,更复杂的社会空间记忆的神经基础尚不清楚。在这里,我们报告了厌恶性社交体验的空间和社交内容需要不同的海马区。在雌性和雄性小鼠中,背侧 CA1(dCA1)锥体神经元介导了对厌恶位置的记忆,而 dCA2 锥体神经元则能区分与威胁相关(CS+)和与安全相关(CS-)的同种异体。在编码或回忆试验中沉默dCA2会破坏社会恐惧辨别记忆,导致对CS+和CS-小鼠都产生恐惧反应。钙成像显示,厌恶体验加强并稳定了CS+和CS-小鼠的dCA2表征,将社会价值的抽象表征纳入了社会身份表征。因此,在厌恶性社会偶发经历中,dCA2既有助于社会新奇感的检测,也有助于适应性地区分与威胁相关的个体和与安全相关的个体。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


