Farm-dust mediated protection of childhood asthma: Mass cytometry reveals novel cellular regulation
Abstract
Background
Farm-dust mediated asthma protection in childhood was replicated in numerous epidemiological studies. Central immune mechanisms are not fully understood. This exploratory study aimed to disentangle underlying immunological regulation of farm-dust mediated protection in peripheral blood on a single-cell level.
Methods
Single-cell protein expression of in vitro farm-dust stimulated and unstimulated cells from allergic asthmatics and healthy controls were measured using mass cytometry. Analysis of innate and adaptive cellular proportions (linear regression) and T-cell proliferation was performed. Functional marker intensity was investigated using Earth Mover's Distance and the Monte Carlo permutation test.
Results
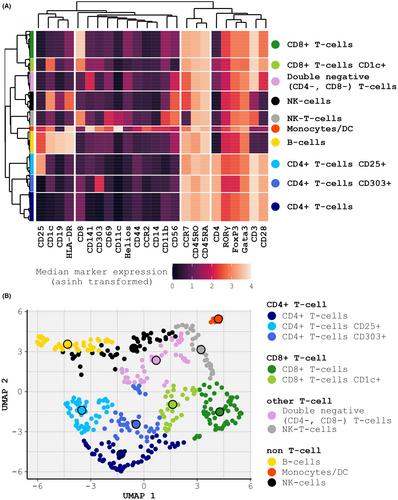
Farm-dust stimulation induced cell type-specific regulation: Key-features of farm-dust stimulation comprised opposing regulation of immune-cell frequencies (downregulated innate cell populations (monocytes/DCs (p < .001), NK-cells (p < .05)) and upregulated adaptive populations (B-cells, CD4+ T-cells (both p < .05)), reduced CD4+ CD25− T-cell proliferation, and differential cell type-specific functional marker expression. Following stimulation, functional marker analysis revealed induced activation (CD25) in T-cells and NK-T-cells in both phenotypes even after correction for multiple testing. Cytotoxicity (GZMB) and inflammation (pERK1/2, pp38) related markers were reduced in T-cells exclusively in asthmatic children. Asthma-associated markers (Gata3, RORγ, and HLA-DR) were reduced in T- and innate- cell populations of asthmatics following stimulation. B-cells displayed a phenotypically independent increase of diverse functional markers upon farm-dust stimulation.
Conclusions
This study mimicking in vivo environmental exposure identified a novel profile of immune-regulatory markers using mass cytometry demonstrating decreased asthma-associated markers following farm-dust stimulation. These findings may be key for further studies on asthma prevention in childhood.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


