An enzymatic cascade for high-yield and stereoselective synthesis of 4-fluoro-L-threonine
IF 11.6
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
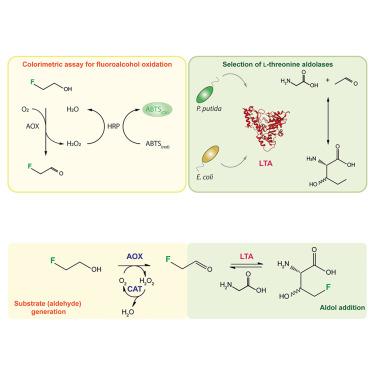
The critical role of fluorine in bioactive molecule design requires selective fluorination methods for synthesizing novel building blocks, such as fluorinated amino acids. Here, we focused on L-threonine aldolases (LTAs), enzymes that mediate reversible aldol additions to the α carbon of glycine. Their C–C bond formation ability and substrate flexibility make these enzymes ideal catalysts for fluorine biocatalysis. We harnessed the promiscuous activity of the LTAs isolated from either Escherichia coli or Pseudomonas putida on 2-fluoroacetaldehyde in a two-step enzymatic cascade for efficient 4-fluoro-L-threonine synthesis. By implementing 2-fluoroethanol as the primary fluorodonor in these cascades, we demonstrated that the LTA enzyme isolated from P. putida mediates a high 4-fluoro-L-threonine yield (>90%) while displaying stereoselectivity for the L-syn form.
高产率和立体选择性合成 4-氟-L-苏氨酸的酶级联反应
氟在生物活性分子设计中的关键作用要求采用选择性氟化方法来合成新型构件,如氟化氨基酸。在这里,我们重点研究了 L-苏氨酸醛醇酶(LTAs),这种酶能介导甘氨酸 α 碳的可逆醛醇加成。它们的 C-C 键形成能力和底物灵活性使这些酶成为氟生物催化的理想催化剂。我们利用从大肠杆菌或假单胞菌中分离出来的 LTAs 在 2-氟乙醛上的杂化活性,通过两步酶级联法高效合成 4-氟-L-苏氨酸。通过在这些级联中使用 2-氟乙醇作为主要的含氟质子,我们证明了从普氏假单胞菌中分离出来的 LTA 酶能介导高产率(90%)的 4-氟-L-苏氨酸,同时对 L-syn 形式显示出立体选择性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


