Promoting effect of interfacial hole accumulation on photoelectrochemical water oxidation in BiVO4 and Mo-doped BiVO4
引用次数: 0
Abstract
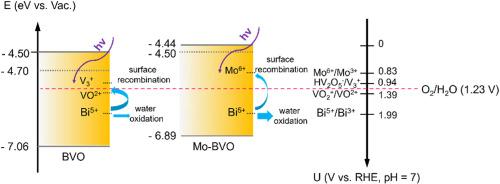
Hole transfer at the semiconductor-electrolyte interface is a key elementary process in (photo)electrochemical (PEC) water oxidation. However, up to now, a detailed understanding of the hole transfer and the influence of surface hole density on PEC water oxidation kinetics is lacking. In this work, we propose a model for the first time in which the surface accumulated hole density in BiVO4 and Mo-doped BiVO4 samples during water oxidation can be acquired via employing illumination-dependent Mott-Schottky measurements. Based on this model, some results are demonstrated as below: (1) Although the surface hole density increases when increasing light intensity and applied potential, the hole transfer rate remains linearly proportional to surface hole density on a log-log scale. (2) Both water oxidation on BiVO4 and Mo-doped BiVO4 follow first-order reaction kinetics at low surface hole densities, which is in good agreement with literature. (3) We find that water oxidation active sites in both BiVO4 and Mo-doped BiVO4 are very likely to be Bi5+, which are produced by photoexcited or/and electro-induced surface holes, rather than VOx species or Mo6+ due to their insufficient redox potential for water oxidation. (4) Introduction of Mo doping brings about higher OER activity of BiVO4, as it suppresses the recombination rate of surface holes and increases formation of Bi5+. This surface hole model offers a general approach for the quantification of surface hole density in the field of semiconductor photoelectrocatalysis.
界面空穴积累对 BiVO4 和掺杂 Mo 的 BiVO4 中光电化学水氧化的促进作用
半导体-电解质界面上的空穴传输是(光)电化学(PEC)水氧化过程中的一个关键基本过程。然而,迄今为止,人们还缺乏对空穴传输以及表面空穴密度对 PEC 水氧化动力学影响的详细了解。在这项工作中,我们首次提出了一个模型,通过利用与光照相关的 Mott-Schottky 测量方法,可以获得水氧化过程中 BiVO4 和掺钼 BiVO4 样品的表面累积空穴密度。根据这一模型,得出了以下结果:(1) 虽然表面空穴密度随光照强度和外加电位的增加而增加,但空穴传输率仍与表面空穴密度成对数线性关系。(2) 水在 BiVO4 和掺 Mo 的 BiVO4 上的氧化反应在低表面空穴密度时都遵循一阶反应动力学,这与文献报道十分吻合。(3) 我们发现,BiVO4 和掺杂 Mo 的 BiVO4 中的水氧化活性位点很可能是由光激发或/和电诱导表面空穴产生的 Bi5+,而不是 VOx 物种或 Mo6+,因为它们的氧化还原电位不足以进行水氧化。(4) Mo 的掺杂抑制了表面空穴的重组速度,增加了 Bi5+ 的形成,从而提高了 BiVO4 的 OER 活性。这种表面空穴模型为量化半导体光电催化领域的表面空穴密度提供了一种通用方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


