Insights into the separation of chalcopyrite from pyrite in Mg and Ca using gum acacia, xanthomonas campestris and guar gum: An experimental study validated by theoretical investigations
IF 4.9
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
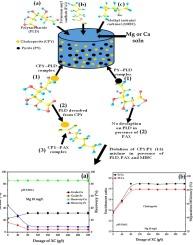
The surface chemistry of chalcopyrite (Cpy) and pyrite (Py) in presence of water containing with Mg and Ca was studied using gum acacia (GA), xanthomonas campestris (XC) and guar gum (GG) as depressants. The adsorption density of XC and GA beyond IEP of Cpy and Py in absence of metal ions was nil, whereas that of GG increased with increase in pH till at pH 9.4. However, in presence of Mg and Ca, the adsorption of XC was higher than that of GA and that of GG was higher onto mineral-metal than onto mineral. Further, the adsorption of XC, GA and GG onto mineral-Mg was more than that onto mineral-Ca and this was due to higher maximum molecular electrostatic potential (MESP) of Mg species (413.3 kcal/mol) relative to Ca species (253 kcal/mol). Additionally, the polysaccharides’ adsorption onto Py-Ca and Py-Mg was higher than onto Cpy-Mg and Cpy-Ca. On Cpy and Py with metal ions, the adsorption of XC and GA decreased with increase in pH attesting to polysaccharides anionicity. Theoretical analysis of complexes formed on Py and Cpy surfaces containing Mg and Ca, and polysaccharides was due to conventional hydrogen bonding, non-covalent and partially covalent bonding. Flotation of synthetic mixture of Py and Cpy (1:1) in presence of Mg at pH 9.5 performed better than other systems yielding Cu grade and recovery of 31.1 % and 85.6 % after 80 g/t of XC. Therefore, XC is a potential Py depressant in presence of Ca and Mg species.
利用刺槐胶、黄单胞菌和瓜尔胶从黄铁矿中分离出镁和钙的黄铜矿的见解:通过理论研究验证的实验研究
以金合欢胶(GA)、黄单胞菌(XC)和瓜尔豆胶(GG)为抑制剂,研究了黄铜矿(Cpy)和黄铁矿(Py)在含有镁和钙的水中的表面化学性质。在没有金属离子的情况下,XC 和 GA 在 Cpy 和 Py 的 IEP 值之外的吸附密度为零,而瓜尔豆胶的吸附密度随着 pH 值的增加而增加,直到 pH 值为 9.4。然而,在存在镁和钙的情况下,XC 的吸附密度高于 GA,而 GG 在矿物-金属上的吸附密度高于在矿物上的吸附密度。此外,XC、GA 和 GG 在矿物-金属(Mg)上的吸附量高于在矿物-金属(Ca)上的吸附量,这是由于 Mg 物种的最大分子静电势(MESP)(413.3 kcal/mol)高于 Ca 物种(253 kcal/mol)。此外,多糖在 Py-Ca 和 Py-Mg 上的吸附力高于在 Cpy-Mg 和 Cpy-Ca 上的吸附力。在含有金属离子的 Cpy 和 Py 上,XC 和 GA 的吸附量随着 pH 值的增加而减少,这证明了多糖的阴离子性。对含有镁和钙的 Py 和 Cpy 表面形成的复合物进行的理论分析表明,多糖是由于传统的氢键、非共价键和部分共价键形成的。在 pH 值为 9.5、有镁存在的条件下,Py 和 Cpy(1:1)合成混合物的浮选效果优于其他系统,80 克/吨 XC 的铜品位和回收率分别为 31.1% 和 85.6%。因此,在钙和镁存在的情况下,XC 是一种潜在的 Py 抑制剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Minerals Engineering
工程技术-工程:化工
CiteScore
8.70
自引率
18.80%
发文量
519
审稿时长
81 days
期刊介绍:
The purpose of the journal is to provide for the rapid publication of topical papers featuring the latest developments in the allied fields of mineral processing and extractive metallurgy. Its wide ranging coverage of research and practical (operating) topics includes physical separation methods, such as comminution, flotation concentration and dewatering, chemical methods such as bio-, hydro-, and electro-metallurgy, analytical techniques, process control, simulation and instrumentation, and mineralogical aspects of processing. Environmental issues, particularly those pertaining to sustainable development, will also be strongly covered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


