Prognostic impact of EGFR expression and immunohistochemistry-based “molecular classification” in bladder cancer
IF 1.4
4区 医学
Q3 PATHOLOGY
引用次数: 0
Abstract
Recent genomic studies emphasize the necessity of molecular classification to reflect diverse clinical and pathological characteristics of bladder cancer. Immunohistochemically bladder cancer can be classified into molecular subtypes, including basal, luminal, and p53-like subtypes. Epidermal growth factor receptor (EGFR) is frequently expressed in basal-type bladder cancers and is associated with poor prognosis. In our study, 88 urothelial carcinoma cases were retrospectively analyzed, molecularly subtyped using CK5/6, GATA3, p16 immunohistochemistry and examined for EGFR expressions as well as clinical and histopathological features. Tumor cell scores ≥20 % considered positive, classifying cases as luminal (GATA3-positive), basal (CK5/6-positive), double-positive (both-positive), or double-negative (both-negative). Further division of luminal and basal cases was based on p16 status: luminal-p53 or basal-p53 (p16-positive) and luminal-non-p53 or basal-non-p53 (p16-negative). Among the cases, 4 (4 %) were double-negative, 48 (55 %) luminal-non-p53, 21 (24 %) luminal-p53, 5 (6 %) basal-non-p53, 3 (3 %) basal-p53, and 7 (8 %) double-positive. Our findings revealed that basal-non-p53 type bladder cancer is associated with poor prognosis, muscle invasion, and high-grade cytology. Basal-p53 and double-negative types exhibited less aggressive features compared to basal-non-p53 types, with associations observed with lamina propria invasion and high-grade cytology. Luminal-p53 type demonstrated higher recurrence rates. Luminal-non-p53 type displayed the least aggressive characteristics, often associated with papillary histopathology. EGFR expression was found to be high in basal-non-p53 type and was further correlated with adverse prognostic indicators, lamina propria invasion, and high-grade cytology. The identification of molecular subtypes and EGFR expression through immunohistochemistry, alongside traditional bladder cancer classifications, enhances tumor behavior prediction and supports effective clinical management.
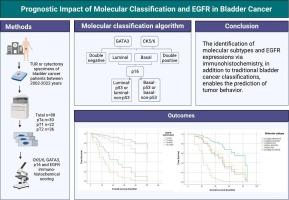
表皮生长因子受体表达和基于免疫组化的 "分子分类 "对膀胱癌预后的影响
最近的基因组研究强调,有必要进行分子分类,以反映膀胱癌不同的临床和病理特征。免疫组化法可将膀胱癌分为分子亚型,包括基底型、管腔型和 p53 样亚型。表皮生长因子受体(EGFR)常在基底型膀胱癌中表达,与预后不良有关。我们的研究对 88 例尿路上皮癌病例进行了回顾性分析,利用 CK5/6、GATA3、p16 免疫组织化学法进行了分子亚型划分,并检查了表皮生长因子受体的表达以及临床和组织病理学特征。肿瘤细胞评分≥20%为阳性,将病例分为管腔型(GATA3阳性)、基底型(CK5/6阳性)、双阳性(均阳性)或双阴性(均阴性)。根据 p16 状态进一步划分管腔和基底病例:管腔-p53 或基底-p53(p16 阳性)和管腔-非 p53 或基底-非 p53(p16 阴性)。其中,4 例(4%)为双阴性,48 例(55%)为管腔-非 p53,21 例(24%)为管腔-p53,5 例(6%)为基底-非 p53,3 例(3%)为基底-p53,7 例(8%)为双阳性。我们的研究结果表明,基底-非 p53 型膀胱癌与预后不良、肌肉侵犯和高级别细胞学相关。与基底-非 53 型膀胱癌相比,基底-53 型和双阴性型膀胱癌的侵袭性较低,与固有层浸润和高级别细胞学相关。基底-p53 型的复发率较高。基底-非p53型的侵袭性最小,通常与乳头状组织病理学有关。研究发现,表皮生长因子受体(EGFR)的表达在基底-非p53型中较高,并与不良预后指标、固有层侵犯和高级别细胞学进一步相关。通过免疫组化鉴定分子亚型和表皮生长因子受体表达,再加上传统的膀胱癌分类,可提高肿瘤行为预测能力,支持有效的临床治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.90
自引率
5.00%
发文量
149
审稿时长
26 days
期刊介绍:
A peer-reviewed journal devoted to the publication of articles dealing with traditional morphologic studies using standard diagnostic techniques and stressing clinicopathological correlations and scientific observation of relevance to the daily practice of pathology. Special features include pathologic-radiologic correlations and pathologic-cytologic correlations.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


