Thermodynamics of the formation of polyynes and aromatic species from methane and acetylene
IF 1.7
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The Gibbs free energies of the formation of several polyynes (C6H2, C10H2 and C16H2) and aromatic species (C6H6, C10H8 and C16H10) from methane and acetylene at temperatures of 1000–2600 K and atmospheric pressure were obtained by quantum chemical calculations using the RI-MP2 method in the ORCA open source software. At lower temperatures, aromatic species form more readily than polyynes, while at temperatures >2200 K the trend reverses and polyyne formation becomes predominant.
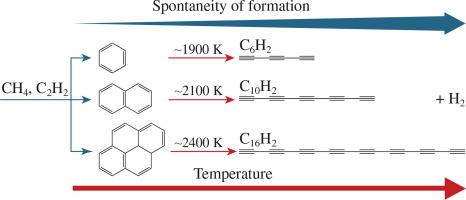
甲烷和乙炔生成多炔和芳香族物质的热力学
利用 ORCA 开放源码软件中的 RI-MP2 方法,通过量子化学计算获得了甲烷和乙炔在 1000-2600K、大气压力下形成几种多炔(C6H2、C10H2 和 C16H2)和芳香族(C6H6、C10H8 和 C16H10)的吉布斯自由能。在较低温度下,芳香族物质比聚炔更容易形成,而在温度为 2200 K 时,这一趋势发生逆转,聚炔的形成占主导地位。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Mendeleev Communications
化学-化学综合
CiteScore
3.00
自引率
21.10%
发文量
226
审稿时长
4-8 weeks
期刊介绍:
Mendeleev Communications is the journal of the Russian Academy of Sciences, launched jointly by the Academy of Sciences of the USSR and the Royal Society of Chemistry (United Kingdom) in 1991. Starting from 1st January 2007, Elsevier is the new publishing partner of Mendeleev Communications.
Mendeleev Communications publishes short communications in chemistry. The journal primarily features papers from the Russian Federation and the other states of the former USSR. However, it also includes papers by authors from other parts of the world. Mendeleev Communications is not a translated journal, but instead is published directly in English. The International Editorial Board is composed of eminent scientists who provide advice on refereeing policy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


