Visible-light-mediated cyclopropanation reactions of 3-diazooxindoles with olefins
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
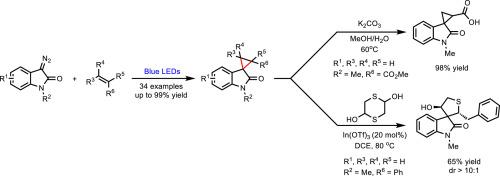
Visible-light-mediated metal-free cyclopropanation reaction of diazo compounds with different olefins or arenes have been fully developed in recent years. However, electron-deficient olefins still could not be well tolerated in these reactions. Here, by using 3-diazooxindole as the carbene precursor, the visible-light-mediated cyclopropanation reaction of diazo compounds with electron-deficient olefins was successfully developed. The electron-rich olefins were also well tolerated in the reactions. A series of spirocyclopropyloxindoles were synthesized in moderate to high yields under mild, metal-free conditions. Mechanism studies reveal that both singlet and triplet carbenes are probably involved in the reaction.
可见光介导的 3-重氮氧化吲哚与烯烃的环丙烷化反应
近年来,以可见光为介导的重氮化合物与不同烯烃或炔烃的无金属环丙烷化反应得到了充分发展。然而,缺电子的烯烃在这些反应中仍然不能很好地耐受。本文以 3-重氮吲哚为碳烯前体,成功开发了重氮化合物与缺电子烯烃在可见光介导下的环丙烷化反应。富电子烯烃在反应中也有很好的耐受性。在温和、无金属的条件下合成了一系列螺环丙基吲哚,产率从中等到较高。机理研究表明,单碳原子和三碳原子可能都参与了反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


