Assembling phthalaldehyde and o-aminochalcones by Pd(II) catalyzed tandem reaction for synthesizing isoindoloindonole fluorophores
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
One-pot synthesis of isoindoloindonoles having various auxochrome, from easily available starting materials phthalaldehyde and o-aminochalcone, involving palladium(II) acetate catalysis in acetic acid is reported. A mechanism based on the formation of 2-aryl-2H-isoindol-1-ol (22) intermediate is proposed for the formation of the tetracyclic core of isoindoloindonoles. The possible intramolecular cyclization via the direct participation of the carbonyl vs the adjacent αβ-conjugated double bond was evaluated. Thus, a detailed study on how the presence of electronically different groups at the β position of chalcones affects the outcome of the cyclization reaction and the photophysical properties of the resulting isoindoloindolones is presented here. Furthermore, the core structure was determined using NMR, mass and single-crystal X-ray diffraction analysis.
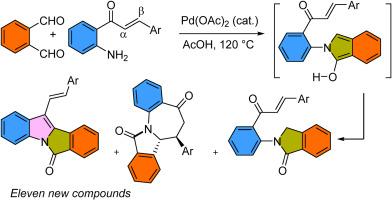
通过钯(II)催化串联反应组装邻苯二甲醛和邻氨基查耳酮以合成异吲哚吲哚荧光团
本研究报道了在乙酸中通过乙酸钯(II)催化,从易于获得的起始原料邻苯二甲醛和邻氨基查尔酮出发,一步法合成具有各种辅助色素的异吲哚吲哚类化合物。提出了一种基于 2-芳基-2H-异吲哚-1-醇 (22) 中间体形成的机制,用于形成异吲哚酮类化合物的四环核心。通过羰基与邻近的 αβ 共轭双键的直接参与,对分子内环化的可能性进行了评估。因此,本文详细研究了在查耳酮的 β 位上存在电子不同的基团如何影响环化反应的结果以及所得异吲哚吲哚酮的光物理特性。此外,还利用核磁共振、质量和单晶 X 射线衍射分析确定了核心结构。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


