Organocatalytic domino formation of (3R,3aS,9bR)-configured 3-aryl-3a-benzamido-1,3a,4,9b-tetrahydrochromeno[4,3-b]pyrroles in carbon dioxide medium
IF 1.7
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Asymmetric cycloaddition/intramolecular rearrangement domino reaction of 2-(2-hydroxybenzylideneamino)- malonates with 4-arylidene-2-phenyloxazol-5(4H)-ones can be efficiently carried out in sub- or supercritical carbon dioxide to afford (3R,3aS,9bR)-3-aryl-3a-benzamido-4-oxo-1,3a,4,9b-tetrahydrochromeno[4,3-b]pyrrole-2,2(3H)- dicarboxylates in high yields with up to 99% ee. Excellent stereoinduction is provided in this process by the use of bifunctional hybrid organocatalyst consisting of squaramide (thiourea) and chiral tertiary amine units.
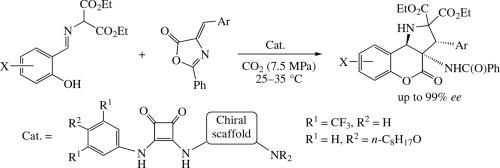
在二氧化碳介质中有机催化形成 (3R,3aS,9bR)-构型 3-芳基-3a-苯甲酰胺基-1,3a,4,9b-四氢苯并吡喃并[4,3-b]吡咯的多米诺效应
2-(2-hydroxybenzylideneamino)- malonates 与 4-arylidene-2-phenyloxazol-5(4H)-ones 的不对称环加成/分子内重排多米诺反应可在亚临界或超临界二氧化碳中高效进行,从而得到 (3R.3aS,9bR)-3-芳基-3a-苯甲酰胺基-4-氧代-1,3a,4,9b-四氢苯并吡咯-2,3-酮、3aS,9bR)-3-芳基-3a-苯甲酰胺基-4-氧代-1,3a,4,9b-四氢苯并吡咯并[4,3-b]吡咯-2,2(3H)-二甲酸酯,收率高,ee 值高达 99%。通过使用由方酰胺(硫脲)和手性叔胺单元组成的双功能杂化有机催化剂,该工艺具有极佳的立体诱导性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Mendeleev Communications
化学-化学综合
CiteScore
3.00
自引率
21.10%
发文量
226
审稿时长
4-8 weeks
期刊介绍:
Mendeleev Communications is the journal of the Russian Academy of Sciences, launched jointly by the Academy of Sciences of the USSR and the Royal Society of Chemistry (United Kingdom) in 1991. Starting from 1st January 2007, Elsevier is the new publishing partner of Mendeleev Communications.
Mendeleev Communications publishes short communications in chemistry. The journal primarily features papers from the Russian Federation and the other states of the former USSR. However, it also includes papers by authors from other parts of the world. Mendeleev Communications is not a translated journal, but instead is published directly in English. The International Editorial Board is composed of eminent scientists who provide advice on refereeing policy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


