Annulation of 1-methylisoquinoline with pyrrolylacetylenic ketones: catalyst-free stereoselective synthesis of (E)-acylethenylpyrrolo[1’,2’:3,4]imidazo[2,1-a]isoquinolines
IF 1.7
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
1-Methylisoquinoline undergoes stereoselective annulation with pyrrolylacetylenic ketones (MeCN, 80–82 °C) to provide (E)-acylethenylpyrrolo[1’,2’:3,4]imidazo[2,1-a]isoquinolines in up to 92% yield. In the case of 5-arylpyrrolylacetylenic ketones, instead of the above cyclization, the dimerization of the starting ketones to give dipyrrolopyrazines in 38 and 39% yields occurs.
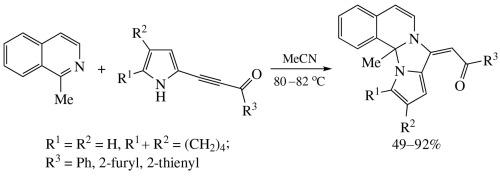
1-甲基异喹啉与吡咯乙炔基酮的环化反应:无催化剂立体选择性合成(E)-乙酰乙烯基吡咯并[1',2':3,4]咪唑并[2,1-a]异喹啉类化合物
1-甲基异喹啉与吡咯乙炔基酮发生立体选择性环化反应(MeCN,80-82 °C),得到(E)-乙烯基吡咯并[1',2':3,4]咪唑并[2,1-a]异喹啉,收率高达 92%。在 5-芳基吡咯乙炔基酮的情况下,起始酮二聚生成二吡咯并吡嗪,而不是上述的环化反应,收率分别为 38% 和 39%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Mendeleev Communications
化学-化学综合
CiteScore
3.00
自引率
21.10%
发文量
226
审稿时长
4-8 weeks
期刊介绍:
Mendeleev Communications is the journal of the Russian Academy of Sciences, launched jointly by the Academy of Sciences of the USSR and the Royal Society of Chemistry (United Kingdom) in 1991. Starting from 1st January 2007, Elsevier is the new publishing partner of Mendeleev Communications.
Mendeleev Communications publishes short communications in chemistry. The journal primarily features papers from the Russian Federation and the other states of the former USSR. However, it also includes papers by authors from other parts of the world. Mendeleev Communications is not a translated journal, but instead is published directly in English. The International Editorial Board is composed of eminent scientists who provide advice on refereeing policy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


