Facile one-pot synthesis of Bi2S3 nanorod @ N, S co-doped carbon composite for high performance lithium ion batteries
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
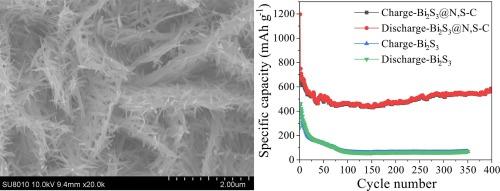
Bismuth sulfide is favoured in lithium ion batteries due to its high specific capacity of 625 mAh/g. However, the Bi2S3 anode faces severe volume expansion problems during the lithium intercalation process, resulting in continuous electrode fragmentation and rapid degradation of lithium storage performance. In this study, Bi2S3 nanorod@N, S co-doped carbon composite prepared by a simple sintering method was used as the anode material for lithium ion batteries. 1D Bi2S3 nanorods with a length of 1 μm and a diameter of 50 nm were loaded in situ on 2D N, S co-doped carbon nanosheets. This unique structure can not only alleviate the volume change of bismuth sulfide, but also effectively shorten the diffusion distance of lithium ions, thereby improving the cycling stability and rate capability at the same time. The discharge capacity of Bi2S3@N, S![]() C remained at 583.4 mAh g –1 after 400 cycles at 0.5 A g–1. Even at a high current density of 2 A/g, the discharge capacity of Bi2S3@N, S
C remained at 583.4 mAh g –1 after 400 cycles at 0.5 A g–1. Even at a high current density of 2 A/g, the discharge capacity of Bi2S3@N, S![]() C still reached 374.3 mAh g –1. This simple method also can be extended to the preparation of other metal sulfide composites.
C still reached 374.3 mAh g –1. This simple method also can be extended to the preparation of other metal sulfide composites.
用于高性能锂离子电池的 Bi2S3 纳米棒 @ N、S 共掺杂碳复合材料的简单单锅合成
硫化铋的比容量高达 625 mAh/g,因此在锂离子电池中备受青睐。然而,Bi2S3 负极在锂插层过程中面临严重的体积膨胀问题,导致电极不断碎裂,储锂性能迅速下降。本研究采用简单的烧结方法制备了 Bi2S3 纳米棒@N、S 共掺杂碳复合材料作为锂离子电池的负极材料。长度为 1 μm、直径为 50 nm 的一维 Bi2S3 纳米棒被原位负载在二维 N、S 共掺杂碳纳米片上。这种独特的结构不仅能缓解硫化铋的体积变化,还能有效缩短锂离子的扩散距离,从而同时提高循环稳定性和速率能力。在 0.5 A g-1 下循环 400 次后,Bi2S3@N, SC 的放电容量仍保持在 583.4 mAh g -1 的水平。即使在 2 A/g 的高电流密度下,Bi2S3@N, SC 的放电容量仍然达到 374.3 mAh g -1 。这种简单的方法还可以扩展到其他金属硫化物复合材料的制备。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


