Green synthesis of nanoscale zero-valent iron impregnated walnut shell biochar as efficient adsorbent for metal(loid)s purification: Performance and mechanism insight
IF 6.3
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The escalation of metal(loid) contamination in global water poses severe risks to ecological environment safety. Meeting the ‘pollution control with waste’ strategy, we utilized eco-friendly waste tea leaf extract and walnut shell to first fabricate a greenly synthesized nanoscale zero-valent iron impregnated walnut shell biochar (G-nZVI/WSB) for the simultaneous sequestration of cadmium (Cd), lead (Pb), and arsenic (As) from aqueous systems. Since the interactions among these metal(loid)s during adsorption on G-nZVI/WSB and their corresponding mechanisms remain unclear, we conducted a series of batch experiments coupled with characterization techniques. The optimal pyrolysis temperature and Fe/C ratio for G-nZVI/WSB were determined to be 650 °C and 20 %, respectively. Under optimal conditions (contact time = 24 h, pH = 6), G-nZVI/WSB exhibited theoretical maximum adsorption capacities for Cd(II), Pb(II), and As(III) of 52.96, 135.1, and 8.414 mg g−1, respectively, which were significantly higher than those of WSB carrier. In single and combined systems, the monolayer and chemical adsorption of metal(loid)s were predominant, with their competitive affinity for active sites following the order of Pb(II) > Cd(II) > As(III). Characterization analyses using FTIR, SEM, XRD, and XPS confirmed the specific adsorption mechanisms of G-nZVI/WSB for these metal(loid)s, including cation-π interactions, coprecipitation, oxidation, and coordinated complexation. Notably, G-nZVI/WSB exhibited excellent adsorption stability over five cycles of adsorption-desorption, which achieved the efficient purification of multi-contaminated actual wastewaters to meet the standards for wastewater discharge or drinking water. These findings demonstrate the significant potential of G-nZVI/WSB as an eco-friendly and promising nanocomposite for treating metal(loid)-contaminated wastewater.
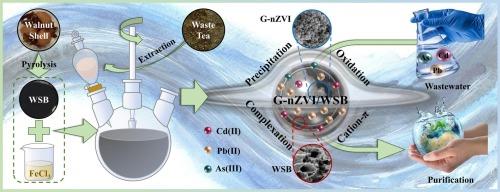
纳米级零价铁浸渍核桃壳生物炭的绿色合成,作为金属(loid)净化的高效吸附剂:性能与机理洞察
全球水体中金属(loid)污染的加剧对生态环境安全构成了严重威胁。为实现 "变废为宝 "的污染控制策略,我们利用环保型废弃茶叶提取物和核桃壳,首次制备出一种绿色合成的纳米级零价铁浸渍核桃壳生物炭(G-nZVI/WSB),用于同时吸附水体中的镉(Cd)、铅(Pb)和砷(As)。由于这些金属(loid)在 G-nZVI/WSB 上吸附过程中的相互作用及其相应机制尚不清楚,我们结合表征技术进行了一系列批量实验。结果表明,G-nZVI/WSB 的最佳热解温度和铁/碳比分别为 650 °C 和 20%。在最佳条件下(接触时间 = 24 小时,pH = 6),G-nZVI/WSB 对 Cd(II)、Pb(II)和 As(III) 的理论最大吸附容量分别为 52.96、135.1 和 8.414 mg g-1,明显高于 WSB 载体。在单一和组合体系中,金属(loid)的单层吸附和化学吸附占主导地位,它们对活性位点的竞争亲和力依次为铅(II)> 镉(II)> 亚砷(III)。利用傅立叶变换红外光谱、扫描电镜、XRD 和 XPS 进行的表征分析证实了 G-nZVI/WSB 对这些金属(loid)的特定吸附机制,包括阳离子-π 相互作用、共沉淀、氧化和配位络合。值得注意的是,G-nZVI/WSB 在五个吸附-解吸循环中表现出优异的吸附稳定性,实现了对多种污染的实际废水的高效净化,达到了废水排放或饮用水的标准。这些研究结果表明,G-nZVI/WSB 作为一种生态友好、前景广阔的纳米复合材料,在处理金属(loid)污染废水方面具有巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of water process engineering
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
10.70
自引率
8.60%
发文量
846
审稿时长
24 days
期刊介绍:
The Journal of Water Process Engineering aims to publish refereed, high-quality research papers with significant novelty and impact in all areas of the engineering of water and wastewater processing . Papers on advanced and novel treatment processes and technologies are particularly welcome. The Journal considers papers in areas such as nanotechnology and biotechnology applications in water, novel oxidation and separation processes, membrane processes (except those for desalination) , catalytic processes for the removal of water contaminants, sustainable processes, water reuse and recycling, water use and wastewater minimization, integrated/hybrid technology, process modeling of water treatment and novel treatment processes. Submissions on the subject of adsorbents, including standard measurements of adsorption kinetics and equilibrium will only be considered if there is a genuine case for novelty and contribution, for example highly novel, sustainable adsorbents and their use: papers on activated carbon-type materials derived from natural matter, or surfactant-modified clays and related minerals, would not fulfil this criterion. The Journal particularly welcomes contributions involving environmentally, economically and socially sustainable technology for water treatment, including those which are energy-efficient, with minimal or no chemical consumption, and capable of water recycling and reuse that minimizes the direct disposal of wastewater to the aquatic environment. Papers that describe novel ideas for solving issues related to water quality and availability are also welcome, as are those that show the transfer of techniques from other disciplines. The Journal will consider papers dealing with processes for various water matrices including drinking water (except desalination), domestic, urban and industrial wastewaters, in addition to their residues. It is expected that the journal will be of particular relevance to chemical and process engineers working in the field. The Journal welcomes Full Text papers, Short Communications, State-of-the-Art Reviews and Letters to Editors and Case Studies
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


