Benching of a real Chinese salt lake environment to achieve lithium ion concentration and osmotic power generation through ionic equilibrium concentration in the forward osmosis process
IF 6.3
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
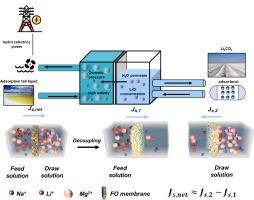
Salt lake brines with high osmotic pressures can be used as natural draw solutions for the Li+ concentration process in forward osmosis. However, the problem of low Li+ concentrations (a few hundred milligrams per liter) has always prevented forward osmosis from being upscaled from the laboratory. Therefore, in this study, a bench-scale 0.5–1.5 g/L Li+ concentration process was carried out, and an equilibrium concentration of approximately 0.2 g/L was reached on the draw solution side. This process was utilized to achieve a low Li+ concentration process with a long cycle recovery of 94 % and no attenuation, the average water flux was 28 LMH, and the equipment was successfully operated 30 times in one month. In addition, the focus of this study was on the effect of the operating conditions on the concentration process. The parameters included the temperature, flow rate, and initial feed solution concentration. The temperature varied among 0, 15, and 25 °C since the salt lake area in China is mostly characterized by high altitudes and cold weather. In addition, this study presents a process to recover the osmotic energy of the draw solution during forward osmosis and increase the Li+ concentration. Our work lays the foundation for the forward osmosis Li+ concentration process in saltwater to move from the laboratory to large-scale production.
利用真实的中国盐湖环境,在正渗透过程中通过离子平衡浓缩实现锂离子浓缩和渗透发电
具有高渗透压的盐湖卤水可用作正向渗透中 Li+ 浓缩过程的天然汲取溶液。然而,低 Li+ 浓度(每升几百毫克)的问题一直阻碍着正渗透技术在实验室中的推广。因此,在本研究中,进行了 0.5-1.5 克/升 Li+ 的台式浓缩过程,并在汲取溶液一侧达到了约 0.2 克/升的平衡浓度。利用该工艺实现了低 Li+ 浓度工艺,长周期回收率达 94 %,且无衰减,平均水通量为 28 LMH,设备在一个月内成功运行了 30 次。此外,本研究的重点是操作条件对浓缩过程的影响。参数包括温度、流速和初始给料溶液浓度。温度在 0、15 和 25 °C 之间变化,因为中国盐湖地区大多海拔较高,天气寒冷。此外,本研究还提出了一种在正向渗透过程中回收汲取溶液渗透能并提高 Li+ 浓度的工艺。我们的工作为盐水中的正渗透 Li+ 浓缩工艺从实验室走向大规模生产奠定了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of water process engineering
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
10.70
自引率
8.60%
发文量
846
审稿时长
24 days
期刊介绍:
The Journal of Water Process Engineering aims to publish refereed, high-quality research papers with significant novelty and impact in all areas of the engineering of water and wastewater processing . Papers on advanced and novel treatment processes and technologies are particularly welcome. The Journal considers papers in areas such as nanotechnology and biotechnology applications in water, novel oxidation and separation processes, membrane processes (except those for desalination) , catalytic processes for the removal of water contaminants, sustainable processes, water reuse and recycling, water use and wastewater minimization, integrated/hybrid technology, process modeling of water treatment and novel treatment processes. Submissions on the subject of adsorbents, including standard measurements of adsorption kinetics and equilibrium will only be considered if there is a genuine case for novelty and contribution, for example highly novel, sustainable adsorbents and their use: papers on activated carbon-type materials derived from natural matter, or surfactant-modified clays and related minerals, would not fulfil this criterion. The Journal particularly welcomes contributions involving environmentally, economically and socially sustainable technology for water treatment, including those which are energy-efficient, with minimal or no chemical consumption, and capable of water recycling and reuse that minimizes the direct disposal of wastewater to the aquatic environment. Papers that describe novel ideas for solving issues related to water quality and availability are also welcome, as are those that show the transfer of techniques from other disciplines. The Journal will consider papers dealing with processes for various water matrices including drinking water (except desalination), domestic, urban and industrial wastewaters, in addition to their residues. It is expected that the journal will be of particular relevance to chemical and process engineers working in the field. The Journal welcomes Full Text papers, Short Communications, State-of-the-Art Reviews and Letters to Editors and Case Studies
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


