Synthesis, and molecular docking studies of novel 1,2,3-triazoles-linked pyrazole carboxamides as significant anti-microbial and anti-cancer agents
IF 2.5
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
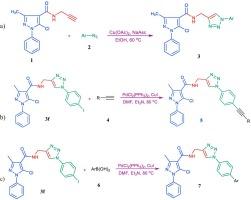
This paper presents the design, synthesis, and evaluation of a series of novel 1,2,3-triazole-pyrazole hybrids. These compounds were specifically developed to assess their cytotoxic activities against various microorganisms and cancer cell lines, namely MCF7 and OVCAR3. The results of the in vitro testing revealed that several compounds exhibited significant inhibitory effects on both microorganisms and cancer cells. Notably, compound 7e demonstrated exceptional antibacterial activity against E. coli, with an effective concentration range of 0.778 ± 0.009 µM. Additionally; compound 7c displayed the highest inhibitory effect on P. aeruginosa and C. albicans, with an effective concentration of 0.743 ± 0.005 µM. In terms of cytotoxicity, compound 7a showed the most potent effect against MCF7 cells, with an IC50 value of 0.304 ± 0.006 µM. Furthermore, compound 5b exhibited the highest cytotoxicity against OVCAR3 cells, with a concentration of 0.233 ± 0.001 µM. These findings indicate the potential of the synthesized 1,2,3-triazole-pyrazole hybrids as promising candidates for further investigation as antimicrobial and anticancer agents. Molecular docking was employed to explore the binding mode between the synthesized and developed compounds and their respective targets. The active site of the receptor displayed diverse hydrophilic and hydrophobic interactions, underscoring the significant potential of the synthesized chemical compounds. To ensure further validation, an analysis of absorption, distribution, metabolism, excretion, and toxicity (ADMET) was conducted on the synthesized pyrazole carboxamide derivatives. The outcomes of this study strongly confirm that the proposed compounds are potent against different microorganisms.
新型 1,2,3-三唑连环吡唑羧酰胺作为重要抗微生物剂和抗癌剂的合成与分子对接研究
本文介绍了一系列新型 1,2,3-三唑-吡唑杂化物的设计、合成和评估。开发这些化合物的目的是评估它们对各种微生物和癌细胞株(即 MCF7 和 OVCAR3)的细胞毒活性。体外测试结果表明,几种化合物对微生物和癌细胞都有显著的抑制作用。值得注意的是,化合物 7e 对大肠杆菌具有特殊的抗菌活性,有效浓度范围为 0.778 ± 0.009 µM。此外,化合物 7c 对绿脓杆菌和白僵菌的抑制作用最强,有效浓度为 0.743 ± 0.005 µM。在细胞毒性方面,化合物 7a 对 MCF7 细胞的作用最强,IC50 值为 0.304 ± 0.006 µM。此外,化合物 5b 对 OVCAR3 细胞的细胞毒性最高,浓度为 0.233 ± 0.001 µM。这些研究结果表明,合成的 1,2,3-三唑-吡唑混合物具有作为抗菌剂和抗癌剂进行进一步研究的潜力。分子对接被用来探索合成和开发的化合物与各自靶标的结合模式。受体的活性位点显示出多种亲水和疏水相互作用,凸显了合成化合物的巨大潜力。为确保进一步验证,对合成的吡唑羧酰胺衍生物进行了吸收、分布、代谢、排泄和毒性(ADMET)分析。这项研究的结果有力地证实了所提出的化合物对不同的微生物都有很强的抑制作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


