A novel sandwich ELISA method for quantifying CHI3L1 in blood serum and cerebrospinal fluid multiple sclerosis patients using sustainable photo-irradiated zero-valence gold nanoparticles
IF 2.5
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
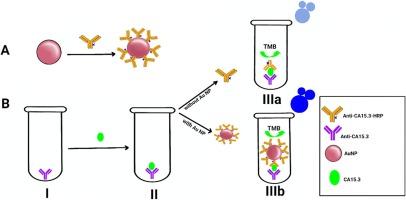
The widespread occurrence of chronic demyelinating MS presents a significant challenge for ongoing research aimed at comprehending its progression, diagnosis, and treatment. In our research, we have devised a novel approach to quantify CHI3L1 (Chitinase-3-like protein 1), which has emerged as a valuable biomarker in MS because of its involvement in inflammation, tissue remodeling, and immune response—crucial factors in regulating damaged nerve cells and preventing their accumulation in the brain, utilizing sandwich ELISA technology. This innovative method primarily depends on gold nanoparticles synthesized through a novel process of photo-irradiation with ultraviolet light. The crystal structure and particle size of sustainable zero-valent gold nanoparticles were measured using X-ray diffraction (XRD), which proved the cubic shape, with a cubic unit cell edge length of 4.0095 Å. The transmission electron microscopy (TEM) and field emission scanning electron microscope (FE-SEM) demonstrated that the distribution of Au NPs is uniform, with an average diameter of 22 nm. The UV–Vis spectrum, displaying a pronounced absorbance peak at a wavelength of 548 nm, suggests the presence of Au NPs. The optimal conditions for the formation of the Au-biotinylated antibody-HRP complex were studied. The optimum pH was 7, the dilution ratio of AuNPs with biotinylated antibody-HRP was 1:3, and the concentration of nanogold was (1 × 10−3 mg/ml) to avoid increasing it. Then a standard curve was drawn for this method and compared to the traditional method, and it was found to be twice as sensitive as the traditional method, with a high accuracy of CV% (3–6 %) as well as a rapid color generation for measurement. This method, based on gold nanoparticles that are considered a carrier for biotinylated antibody-HRP, was applied for the first time to measure CHI3L1 in the sera and cerebrospinal fluid of patients with multiple sclerosis, and the results demonstrated the accuracy and sensitivity of the method. The method has the potential to be an excellent tool for evaluating CHI3L1 and may have applications in other diseases for rapid diagnosis and timely treatment.
利用可持续光辐射零价金纳米粒子的新型夹心 ELISA 方法定量检测多发性硬化症患者血清和脑脊液中的 CHI3L1
慢性脱髓鞘性多发性硬化症的广泛发生给正在进行的旨在了解其进展、诊断和治疗的研究带来了重大挑战。CHI3L1(几丁质酶-3 样蛋白 1)因参与炎症、组织重塑和免疫反应而成为多发性硬化症的重要生物标志物。这种创新方法主要依赖于通过紫外线光照射新工艺合成的金纳米粒子。透射电子显微镜(TEM)和场发射扫描电子显微镜(FE-SEM)表明,金纳米粒子分布均匀,平均直径为 22 纳米。紫外可见光谱在 548 纳米波长处显示出明显的吸光峰,表明金氧化物的存在。研究了形成 Au 生物素化抗体-HRP 复合物的最佳条件。最佳 pH 值为 7,AuNPs 与生物素化抗体-HRP 的稀释比为 1:3,纳米金的浓度为(1 × 10-3 mg/ml),以避免增加纳米金的浓度。然后绘制了该方法的标准曲线,并与传统方法进行了比较,结果发现该方法的灵敏度是传统方法的两倍,CV%(3-6 %)的准确度高,而且测量时可快速显色。该方法以金纳米粒子为生物素化抗体-HRP的载体,首次应用于测定多发性硬化症患者血清和脑脊液中的CHI3L1,结果表明了该方法的准确性和灵敏度。该方法有望成为评估 CHI3L1 的绝佳工具,并可应用于其他疾病的快速诊断和及时治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


