Differential interaction modes of As(III)/As(V) with microbial cell membrane induces opposite effects on organic contaminant biodegradation in groundwater
IF 10.3
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
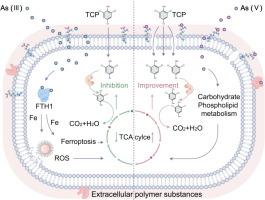
Arsenic, a widespread toxic metalloid in groundwater, derives both from natural geological environment and industrial discharge, is extensively detected to be coexisting with organic contaminants, such as 2,4,6-trichlorophenol (TCP), a prior concerned pollutant. During biological remediation of groundwater, arsenic potentially intervenes microbial behaviors. This study found an opposite interference of arsenic in its two different valences (III and V) on the degradation of TCP by the functional bacteria, Sphingomonas fennica K101. As(III) inhibited TCP degradation in a concentration-dependent manner (from 0.1-10 mg/L), with a maximum inhibition rate of 35.5%, whereas As(V) exhibited promoting effects by 13.8% and 33.2% at 1 mg/L and 10 mg/L, respectively. Employing field emission transmission electron microscopy, quantum chemical calculations, fourier-transform ion cyclotron resonance mass spectrometry and metabolomic analysis, we unveil distinct interactions between cell membranes and arsenic in two valence states. Exposure to As(III) led to significant accumulation of As(III) in the cytoplasm, followed by interaction with intracellular ferritin (ferritin heavy chain 1), releasing iron ions and generating ROS. Subsequently, it induced ferroptosis and disrupted bacterial basal metabolism, thereby inhibiting TCP biodegradation. Oppositely, As(V) bound to a critical component sphingosine and triggered sphingosine polymerization, increasing membrane permeability, which was evidenced by measuring lactate dehydrogenase release. This process facilitated TCP transmembrane permeation by reducing membrane or extracellular secretion resistance. As(V) concurrently upregulated energy metabolism and accelerated TCP degradation. Our study elucidates the influence of prevalent arsenic on biodegradation efficacy, particularly amidst changing redox conditions associated with varying arsenic valences.
As(III)/As(V)与微生物细胞膜的不同相互作用模式对地下水中有机污染物的生物降解产生相反的影响
砷是地下水中一种广泛存在的有毒类金属,来源于自然地质环境和工业排放,被广泛检测到与有机污染物共存,如 2,4,6-三氯苯酚 (TCP),这是一种先前受到关注的污染物。在地下水的生物修复过程中,砷可能会干扰微生物的行为。本研究发现,两种不同价态(III 价和 V 价)的砷对功能细菌 Sphingomonas fennica K101 降解 TCP 的干扰截然相反。砷(III)以浓度依赖的方式(0.1-10 毫克/升)抑制 TCP 降解,最大抑制率为 35.5%,而砷(V)在 1 毫克/升和 10 毫克/升时分别有 13.8% 和 33.2% 的促进作用。利用场发射透射电子显微镜、量子化学计算、傅立叶变换离子回旋共振质谱和代谢组学分析,我们揭示了细胞膜与两种价态砷之间不同的相互作用。暴露于 As(III)后,As(III)在细胞质中大量积累,随后与细胞内的铁蛋白(铁蛋白重链 1)相互作用,释放铁离子并产生 ROS。随后,它诱导了铁蛋白沉积,破坏了细菌的基础代谢,从而抑制了 TCP 的生物降解。相反,As(V)与关键成分鞘磷脂结合,引发鞘磷脂聚合,增加了膜的通透性,通过测量乳酸脱氢酶的释放可以证明这一点。这一过程通过降低膜或细胞外分泌阻力促进了 TCP 跨膜渗透。与此同时,As(V)还能促进能量代谢,加速 TCP 降解。我们的研究阐明了普遍存在的砷对生物降解功效的影响,尤其是在与不同砷价相关的氧化还原条件不断变化的情况下。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environment International
环境科学-环境科学
CiteScore
21.90
自引率
3.40%
发文量
734
审稿时长
2.8 months
期刊介绍:
Environmental Health publishes manuscripts focusing on critical aspects of environmental and occupational medicine, including studies in toxicology and epidemiology, to illuminate the human health implications of exposure to environmental hazards. The journal adopts an open-access model and practices open peer review.
It caters to scientists and practitioners across all environmental science domains, directly or indirectly impacting human health and well-being. With a commitment to enhancing the prevention of environmentally-related health risks, Environmental Health serves as a public health journal for the community and scientists engaged in matters of public health significance concerning the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


