Catalytic copolymerization of carbon dioxide and cyclohexene oxide by a trinuclear cyclohexane-bridged tetradentate Schiff base chromium complex†
IF 4.1
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
Abstract
The development of catalytic systems is a central area of research in carbon dioxide (CO2) and epoxy copolymerization. A novel trinuclear cyclohexane-bridged tetradentate Schiff base chromium complex was synthesized as a catalyst for the ring-opening copolymerization (ROCOP) of CO2 and cyclohexene oxide (CHO), resulting in the formation of poly (cyclohexene carbonate) (PCHC). The impact of polymerization temperature, CO2 pressure, reaction time, and catalyst loading on complex 's polymerization activity was systematically investigated. It was observed that, with the addition of the co-catalyst PPNN3 (PPN = bis(triphenylphosphine)iminium), complex exhibited enhanced catalytic activity for the ROCOP of CO2 and CHO under mild conditions. In contrast, the mononuclear tetradentate Schiff base chromium complex system showed low activity under the same conditions. Compared to complex , complex achieved a higher CHO conversion rate (70%) and 85% PCHC selectivity, with a turnover frequency (TOF) of 419 h−1, which is 5.3 times greater than that of complex . Additionally, the polymer produced by complex had a molecular weight of 13 790 g mol−1, which is higher than that produced by complex (8800 g mol−1) and the commercial Salen CrCl catalyst (9610 g mol−1). By varying the amounts of complex and CHO, PCHC with different molecular weights (6000 g mol−1 to 14 000 g mol−1) and low dispersity can be easily obtained. Notably, the activation energy barrier for polycarbonate formation in the complex system was 21.63 kJ mol−1, compared to 32.88 kJ mol−1 in the complex system.
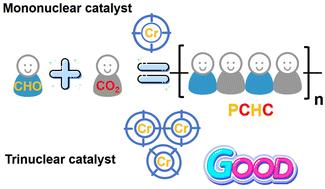
三核环己烷桥接四价席夫碱铬络合物催化二氧化碳与环己烯氧化物的共聚作用
催化体系的开发是二氧化碳(CO2)与环氧树脂共聚的核心研究领域。研究人员合成了一种新型三核环己烷桥接四价席夫碱铬络合物 1,作为二氧化碳和环己烯氧化物(CHO)开环共聚(ROCOP)的催化剂,从而生成聚(环己烯碳酸酯)(PCHC)。系统研究了聚合温度、二氧化碳压力、反应时间和催化剂负载对复合物 1 聚合活性的影响。结果表明,加入助催化剂 PPNN3(PPN= 双(三苯基膦)亚胺)后,复合物 1 在温和条件下对 CO2 和 CHO 的 ROCOP 具有更强的催化活性。相比之下,单核四价席夫碱铬络合物 2 系统在相同条件下的活性较低。与络合物 2 相比,络合物 1 实现了更高的 CHO 转化率(70%)和 85% 的 PCHC 选择性,其周转频率(TOF)为 419 h-1,是络合物 2 的 5.3 倍。此外,复合物 1 生成的聚合物分子量为 13790 克/摩尔,高于复合物 2(8800 克/摩尔)和商用 Salen CrCl 催化剂(9610 克/摩尔)。通过改变复合物 1 和 CHO 的用量,可以很容易地获得不同分子量(6000 克/摩尔至 14000 克/摩尔)和低分散性的 PCHC。值得注意的是,在复合物 1 体系中形成聚碳酸酯的活化能势垒为 21.63 kJ/mol,而在复合物 2 体系中为 32.88 kJ/mol。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


