Acid-labile and non-degradable cross-linked star polymer model networks by aqueous polymerization for in situ encapsulation and release†
IF 4.1
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
Abstract
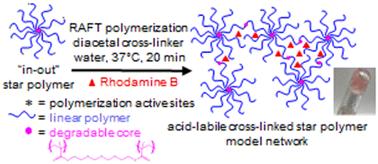
Biocompatible, acid-labile cross-linked star polymer model networks (CSPMNs) have great potential for use in drug delivery. However, a primary complication of this research stems from the prevalence of their synthesis to take place in organic solvents. Herein, to minimize CSPMN potential cytotoxicity, aqueous reversible addition–fragmentation chain transfer polymerization is employed for their synthesis. Initially, “arm-first” star polymers were synthesized in water using a poly[oligo(ethylene glycol) methyl ether methacrylate] (POEGMA) homopolymer and a non-degradable ethylene glycol dimethacrylate or acid-labile diacetal-based bis[(2-methacryloyloxy)ethoxymethyl] ether cross-linker. Subsequently, OEGMA addition resulted in the preparation of “in–out” star polymers (with higher molecular weights) followed by cross-linker addition to form CSPMNs. Rhodamine B dye encapsulation was performed during CSPMN synthesis and its release was observed under biologically relevant conditions. Having shown the effective breakdown of the diacetal-based CSPMNs, their potential for use in drug delivery in low pH environments (i.e. cancerous tumors) is expected to be high.
通过水性聚合实现原位封装和释放的耐酸和不可降解交联星形聚合物模型网络
具有生物相容性的酸性交联星形聚合物模型网络(CSPMNs)在药物输送方面具有巨大的应用潜力。然而,这项研究的一个主要难题是,它们的合成普遍需要在有机溶剂中进行。在此,为了将 CSPMN 潜在的细胞毒性降至最低,我们采用了水性可逆加成-碎片链转移聚合法进行合成。首先,使用聚[低聚(乙二醇)甲基醚甲基丙烯酸酯](POEGMA)均聚物和不可降解的乙二醇二甲基丙烯酸酯或基于酸性二缩醛的双[(2-甲基丙烯酰氧基)乙氧基甲基]醚交联剂在水中合成 "先臂 "星型聚合物。随后,加入 OEGMA,制备出 "内-外 "星形聚合物(分子量较高),再加入交联剂,形成 CSPMN。在 CSPMN 合成过程中对罗丹明 B 染料进行了封装,并在生物相关条件下观察到其释放。由于二缩醛基 CSPMNs 能有效分解,因此有望在低 pH 值环境(如癌症肿瘤)中用于药物输送。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


