Co-Delivery of VEGF siRNA and THPP via Metal–Organic Framework Reverses Cisplatin-Resistant Non-Small Cell Lung Cancer and Inhibits Metastasis through a MUC4 Regulating Mechanism
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Cisplatin resistance significantly impacts the antitumor efficacy of cisplatin chemotherapy and contributes to poor prognosis, including metastasis. In this study, we present the utilization of metal–organic framework (MOF) nanoparticles as the therapeutic component and drug loading scaffold for implementing a ternary combination therapeutic strategy to combat cisplatin-resistant lung cancer and metastasis. Specifically, by engineering MOFs (Cis@MOF-siVEGF) through the self-assembly of THPP as photosensitizer for photodynamic therapy (PDT), along with the incorporation of cisplatin (DDP) and VEGF siRNA (siVEGF), we propose the leverage of photodynamic-induced oxidative damage and gene silencing of the angiogenic factor to reverse cisplatin resistance and sensitize therapeutic potency. Our findings demonstrated that the chemo/photodynamic/antiangiogenic triple combination therapy via Cis@MOF-siVEGF under irradiation effectively inhibits cisplatin-resistant tumor growth and induces abscopal effects. Importantly, molecular mechanistic exploration suggested that MUC4 exerted regulatory effects on governing cancer metastasis, thus representing a potential immunotherapeutic target for cancer intervention. Overall, our study creates a MOFs-based multicomponent delivery platform for complementary therapeutic modules with synergistically enhanced antitumor efficacy and sheds light on potential regulatory mechanisms on cisplatin-resistance cancers.
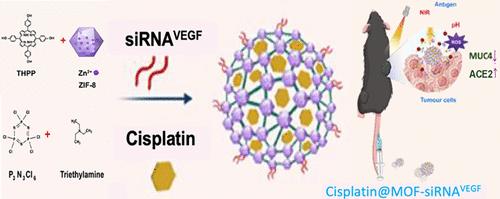
通过金属有机框架联合递送血管内皮生长因子 siRNA 和 THPP 可逆转顺铂耐药的非小细胞肺癌,并通过 MUC4 调节机制抑制转移
顺铂耐药性严重影响顺铂化疗的抗肿瘤疗效,并导致不良预后,包括转移。在本研究中,我们介绍了利用金属有机框架(MOF)纳米颗粒作为治疗成分和药物负载支架,实施三元联合治疗策略,以对抗顺铂耐药肺癌和转移。具体来说,我们通过自组装作为光动力疗法(PDT)光敏剂的THPP,并结合顺铂(DDP)和血管内皮生长因子 siRNA(siVEGF),设计出 MOFs(Cis@MOF-siVEGF),提出利用光动力诱导的氧化损伤和血管生成因子的基因沉默来逆转顺铂耐药性并提高治疗效力。我们的研究结果表明,在照射条件下通过Cis@MOF-siVEGF进行化疗/光动力/血管生成三联疗法能有效抑制顺铂耐药肿瘤的生长并诱导脱落效应。重要的是,分子机理探索表明,MUC4 具有调控癌症转移的作用,因此是一种潜在的癌症干预免疫治疗靶点。总之,我们的研究为具有协同增强抗肿瘤疗效的互补治疗模块创建了一个基于 MOFs 的多组分递送平台,并揭示了顺铂耐药性癌症的潜在调控机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


