Extraction of mandelic acid with tri-octyl-phosphine oxide (TOPO) in different solvents: Equilibrium and neural network analysis
IF 3.2
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
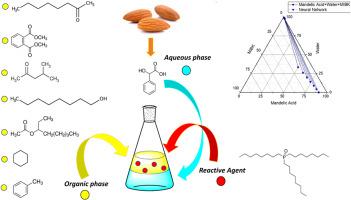
Mandelic acid is an important carboxylic acid used in pharmaceutical industries. It is also important to use it as a purified form. In this study, selective extraction of mandelic acid was done by tri-octyl-phosphine oxide (TOPO) diluted in different solvents such as methyl isobutyl ketone, 1-octanol, octyl acetate, dimethyl phthalate, 2-octanone, cyclohexane and toluene. The high selectivity of mandelic acid from aqueous solution was supported by thermodynamic parameters (loading factor, distribution coefficient, and extraction efficiency). The obtained values for each solvent were applied to the Neural Network Analysis to predict phase equilibrium behaviour in ternary systems. The results showed the highest mandelic acid extraction efficiency (93.65 %) and distribution coefficient (14.74) were attained with the organic phase mixture prepared with MIBK and TOPO. Also, it was found that extraction efficiencies increased with increasing TOPO amount in the medium for all studied solvents.
用三辛基氧化膦(TOPO)在不同溶剂中萃取扁桃酸:平衡和神经网络分析
扁桃酸是一种重要的羧酸,可用于制药业。以纯化的形式使用它也很重要。本研究采用三辛基氧化膦(TOPO)在不同溶剂(如甲基异丁基酮、1-辛醇、乙酸辛酯、邻苯二甲酸二甲酯、2-辛酮、环己烷和甲苯)中稀释的方法对扁桃酸进行选择性萃取。从水溶液中萃取扁桃酸的高选择性得到了热力学参数(负载系数、分配系数和萃取效率)的支持。所获得的每种溶剂的数值都被应用于神经网络分析法,以预测三元体系中的相平衡行为。结果表明,用 MIBK 和 TOPO 制备的有机相混合物的扁桃酸萃取效率(93.65%)和分配系数(14.74)最高。此外,还发现在所有研究溶剂中,萃取效率随着介质中 TOPO 含量的增加而提高。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


