Improved electrochemical performance of the iron-doped NiO nanoparticles at varying calcination temperatures and examination of their supercapacitor applications
IF 3.2
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
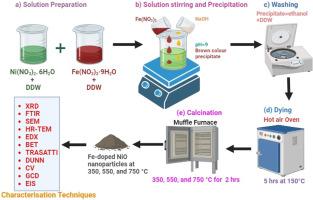
The scientific community is interested in increasing oxide electrochemical characteristics for supercapacitor applications. The present research explores the development of supercapacitor electrodes using Fe-doped NiO nanoparticles synthesised via chemical co-precipitation method with varied calcination temperatures (350, 550, and 750 °C). The key innovation of the work lies in the systematic investigation of the effects of calcination temperature on the electrochemical properties and structural characteristics of the Fe-doped NiO nanoparticles. X-ray diffraction (XRD) analysis revealed a trend of increasing crystallite size with rising temperatures, and optical studies indicated a decreasing trend in the energy band gap from 3.67 eV to 3.23 eV. Fourier transform infrared (FTIR) spectroscopy confirmed the metal-oxygen bond in the molecules. Scanning electron microscopy (SEM) and High-Resolution Transmission Electron Microscopy (HR-TEM) analysis showed the mesoporous spherical morphology of the nanoparticles. Energy-dispersive X-ray spectroscopy (EDX) ensured the samples' elemental composition purity. Brunauer–Emmett–Teller (BET) shows a specific surface area of around 180.8 m2/g was obtained for Fe-doped NiO nanoparticles at 350 °C. Electrochemical tests demonstrated that the Fe-doped NiO electrodes, especially calcined at 350 °C, exhibit superior specific capacitance values (635 F/g) and impressive cycle stability with 93.29 % capacitance retention after 5000 cycles. The present demonstrates the potential of optimizing calcination temperatures to enhance the electrochemical performance and stability of Fe-doped NiO supercapacitor electrodes, marking a significant advancement in supercapacitor technology.
掺铁氧化镍纳米粒子在不同煅烧温度下的电化学性能改进及其超级电容器应用研究
科学界对提高超级电容器应用的氧化物电化学特性很感兴趣。本研究利用化学共沉淀法合成的掺铁氧化镍纳米粒子,在不同的煅烧温度(350、550 和 750 °C)下,探索超级电容器电极的开发。这项工作的主要创新点在于系统地研究了煅烧温度对掺铁氧化镍纳米粒子的电化学特性和结构特征的影响。X 射线衍射(XRD)分析表明,随着温度的升高,晶体尺寸呈增大趋势;光学研究表明,能带隙呈下降趋势,从 3.67 eV 降至 3.23 eV。傅立叶变换红外光谱(FTIR)证实了分子中的金属氧键。扫描电子显微镜(SEM)和高分辨率透射电子显微镜(HR-TEM)分析表明了纳米颗粒的介孔球形形态。能量色散 X 射线光谱(EDX)确保了样品元素组成的纯度。Brunauer-Emmett-Teller (BET) 分析表明,掺铁的氧化镍纳米粒子在 350 °C 时的比表面积约为 180.8 m2/g。电化学测试表明,掺杂铁的氧化镍电极,尤其是在 350 °C 煅烧的电极,显示出卓越的比电容值(635 F/g)和令人印象深刻的循环稳定性,5000 次循环后电容保持率为 93.29%。本研究表明,优化煅烧温度可提高掺铁氧化镍超级电容器电极的电化学性能和稳定性,标志着超级电容器技术的重大进步。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


