A facile and green synthesis of corn cob-based graphene oxide and its modification with corn cob-K2CO3 for efficient removal of methylene blue dye: Adsorption mechanism, isotherm, and kinetic studies
IF 3.2
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
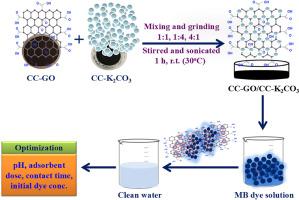
In recent years, the treatment of synthetic dyes has become an environmental concern. In this study, a single step calcination process was used to develop the inventive, simple, and inexpensive adsorbent CC-GO/CC-K2CO3 composite. The composite was employed for the treatment of methylene blue (MB), a cationic dye. Several characterization methods including powder XRD, FTIR, XPS, BET, FESEM, EDX, Raman, and HRTEM techniques were utilized for the analysis of the composite. The surface area and mean pore diameter of CC-GO/CC-K2CO3 were 32.651 m2 g−1 and 3.71 nm, respectively. The adsorption experiment showed that optimal parameters for the removal of MB dye are at an adsorbent dose of 60 mg, initial dye concentration of 80 mg/L, contact time of 150 min, and pH value of 12 at room temperature. Under optimized conditions, CC-GO evidences a removal efficiency of 70.34 ± 1.36 % while after incorporation with CC-K2CO3 the removal capacity sharply increases up to 98.10 ± 0.4 %. Kinetic and isotherm models were used to analyze the removal rate constant and equilibrium adsorption capacity under various adsorption environments. The adsorption study was found to follow the models of pseudo-second order kinetic and Freundlich isotherm. The CC-GO/CC-K2CO3 composite has the maximum adsorption capacity (MAC) of 160.77 mg/g established by the Langmuir isotherm. The prepared composite has demonstrated the capacity to be recycled up to three times with a gradual decrease in its adsorption behavior, exhibiting removal efficiency of 61.66 ± 2.04 %.
A cost estimation study of the composite was also performed to assess its cost effectiveness.
玉米芯基氧化石墨烯的简便绿色合成及其与玉米芯-K2CO3的改性,用于高效去除亚甲基蓝染料:吸附机理、等温线和动力学研究
近年来,合成染料的处理已成为环境问题。在这项研究中,采用单步煅烧工艺开发出了具有创造性、简单且廉价的吸附剂 CC-GO/CC-K2CO3 复合材料。该复合材料用于处理阳离子染料亚甲基蓝(MB)。对该复合材料的分析采用了多种表征方法,包括粉末 XRD、FTIR、XPS、BET、FESEM、EDX、拉曼和 HRTEM 技术。CC-GO/CC-K2CO3 的表面积和平均孔径分别为 32.651 m2 g-1 和 3.71 nm。吸附实验表明,在室温下,吸附剂剂量为 60 毫克、初始染料浓度为 80 毫克/升、接触时间为 150 分钟、pH 值为 12 的条件下,去除 MB 染料的最佳参数为:吸附剂剂量为 60 毫克、初始染料浓度为 80 毫克/升、接触时间为 150 分钟、pH 值为 12。在优化条件下,CC-GO 的去除效率为 70.34 ± 1.36 %,而加入 CC-K2CO3 后,去除能力急剧增加到 98.10 ± 0.4 %。使用动力学和等温线模型分析了各种吸附环境下的去除率常数和平衡吸附容量。发现吸附研究遵循假二阶动力学模型和 Freundlich 等温线模型。根据 Langmuir 等温线,CC-GO/CC-K2CO3 复合材料的最大吸附容量(MAC)为 160.77 mg/g。所制备的复合材料在吸附行为逐渐减弱的情况下可循环使用三次,其去除效率为 61.66 ± 2.04 %。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


