Indole analogs as potential anti-breast cancer agents: Design, synthesis, in-vitro bioevaluation with DFT, molecular docking and ADMET studies
IF 3.2
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
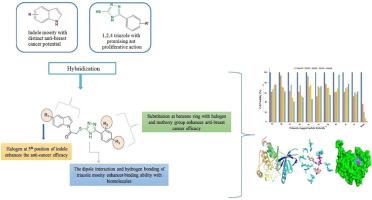
Being a belligerent malignancy, triple-negative breast cancer poses unmet clinical challenges due to lack of targeted therapy, rapid growth rate and metastasis, high heterogeneity, and increased risk of recurrence. Diverse 1-(1H-indol-1-yl)-2-((5-aryl-4H-1,2,4-triazol-3-yl)thio))ethanones have been synthesized in substantial yield (81-87 %) by clubbing 1-(chloroacetyl)indoles with substituted triazoles under refluxing conditions of 4-5 h. The indole integrates demonstrated substantial antitumor efficacy in the MTT assay against the MDA-MB-231 cell line and substituting indole moiety with a halogen (bromo) significantly improves the anti-breast cancer action of the synthesized hybrids compared to the unsubstituted ring. Compound 4i featuring halogen substituent on both the indole and phenyl moieties displayed the highest anticancer action with an IC50 value of 2.121 μM The synthetic variants exhibited a notable binding propensity against the EGFR-TK receptor (PDB ID:1M17). Through in-silico ADMET screening, the pharmacological proclivity of the title compounds has been convicted. The notable bioactivity of the indole hybrids projects them as a potential lead in developing anti-breast cancer medications, especially against TNBC.
作为潜在抗乳腺癌药物的吲哚类似物:设计、合成、使用 DFT 进行体外生物评估、分子对接和 ADMET 研究
三阴性乳腺癌是一种好战的恶性肿瘤,由于缺乏靶向治疗、生长速度快、转移率高、异质性强以及复发风险增加等原因,给临床治疗带来了挑战。通过将 1-(氯乙酰基)吲哚与取代的三唑在 4-5 小时的回流条件下混合,合成了多种 1-(1H-吲哚-1-基)-2-((5-芳基-4H-1,2,4-三唑-3-基)硫))乙酮,收率高达 81-87%。与未取代的环相比,用卤素(溴)取代吲哚分子可显著提高合成杂环的抗乳腺癌作用。在吲哚和苯基上都具有卤素取代基的化合物 4i 显示出最高的抗癌作用,其 IC50 值为 2.121 μM 合成变体对表皮生长因子受体-TK 受体(PDB ID:1M17)具有明显的结合倾向。通过分子内 ADMET 筛选,标题化合物的药理倾向性得到了证实。吲哚类杂交化合物显著的生物活性使其成为开发抗乳腺癌药物(尤其是 TNBC)的潜在先导化合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


