Hydrogel microsphere stem cell encapsulation enhances cardiomyocyte differentiation and functionality in scalable suspension system
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
A reliable suspension-based platform for scaling engineered cardiac tissue (ECT) production from human induced pluripotent stem cells (hiPSCs) is crucial for regenerative therapies. Here, we compared the production and functionality of ECTs formed using our scaffold-based, engineered tissue microsphere differentiation approach with those formed using the prevalent scaffold-free aggregate platform. We utilized a microfluidic system for the rapid (1 million cells/min), high density (30, 40, 60 million cells/ml) encapsulation of hiPSCs within PEG-fibrinogen hydrogel microspheres. HiPSC-laden microspheres and aggregates underwent suspension-based cardiac differentiation in chemically defined media. In comparison to aggregates, microspheres maintained consistent size and shape initially, over time, and within and between batches. Initial size and shape coefficients of variation for microspheres were eight and three times lower, respectively, compared to aggregates. On day 10, microsphere cardiomyocyte (CM) content was 27 % higher and the number of CMs per initial hiPSC was 250 % higher than in aggregates. Contraction and relaxation velocities of microspheres were four and nine times higher than those of aggregates, respectively. Microsphere contractile functionality also improved with culture time, whereas aggregate functionality remained unchanged. Additionally, microspheres displayed improved β-adrenergic signaling responsiveness and uniform calcium transient propagation. Transcriptomic analysis revealed that while both microspheres and aggregates demonstrated similar gene regulation patterns associated with cardiomyocyte differentiation, heart development, cardiac muscle contraction, and sarcomere organization, the microspheres exhibited more pronounced transcriptional changes over time. Taken together, these results highlight the capability of the microsphere platform for scaling up biomanufacturing of ECTs in a suspension-based culture platform.
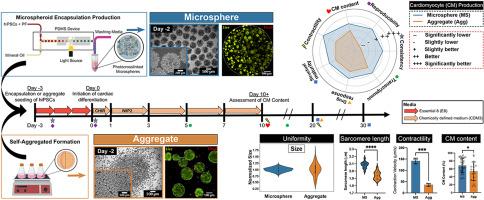
水凝胶微球干细胞封装技术在可扩展的悬浮系统中增强了心肌细胞的分化和功能性
利用人体诱导多能干细胞(hiPSCs)生产工程化心脏组织(ECT)的可靠悬浮平台对再生疗法至关重要。在这里,我们比较了使用我们基于支架的工程组织微球分化方法和使用流行的无支架聚合平台形成的 ECT 的生产和功能。我们利用微流控系统将 hiPSCs 快速(100 万个细胞/分钟)、高密度(3000、4000、6000 万个细胞/毫升)封装在 PEG 纤维蛋白原水凝胶微球中。装载 HiPSC 的微球和聚集体在化学定义的培养基中进行了悬浮心脏分化。与聚集体相比,微球在最初、随着时间的推移、批次内和批次间都能保持一致的大小和形状。与聚集体相比,微球的初始尺寸和形状变异系数分别低八倍和三倍。在第 10 天,微球心肌细胞(CM)含量比聚集体高 27%,每个初始 hiPSC 的 CM 数量比聚集体高 250%。微球的收缩和松弛速度分别是聚集体的四倍和九倍。微球的收缩功能也随着培养时间的延长而提高,而聚集体的功能则保持不变。此外,微球显示出更好的β肾上腺素能信号反应性和均匀的钙瞬态传播。转录组分析表明,虽然微球和聚集体都表现出与心肌细胞分化、心脏发育、心肌收缩和肌节组织相关的类似基因调控模式,但随着时间的推移,微球表现出更明显的转录变化。综上所述,这些结果凸显了微球平台在悬浮培养平台中扩大 ECT 生物制造规模的能力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


