Synthesis of novel skipped diene-3-halocoumarin conjugates as potent anticancer and antibacterial biocompatible agents
IF 2.5
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
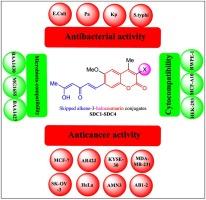
Cancer and bacterial infections are persistent nightmares for human health, which are now aggravated by the development of new types of cancer and pathogenic bacteria, as well as their resistance to prescribed drugs. To be part of a hopeful awakening, this work aimed to synthesize four novel skipped diene-3-halocoumarin conjugates coded SDC1-SDC4 and explore their biosafe functions as anticancer and antibacterial alternatives. In the first step of their synthesis, mequinol reacts with ethyl-2-haloacetoacetate through a H2SO4-catalyzed Pechmann reaction, yielding 3-halo-6-methoxy-4-methylcoumarins. The in situ-generated Vilsmeier-Haack reagent formylated these trifunctionalized coumarins to create their 7-carbaldehyde congeners. Finally, the target conjugates were prepared by condensing the latter compounds with 2,4-pentadione through the modified Claisen-Schmidt reaction. The molecular structures involving their stereochemistry were established for SDC1-SDC4 by analyzing their spectra released from FTIR, 1H NMR, 13C NMR, and HRMS. On the other hand, the anticancer and cytotoxicity of these conjugates were investigated against eight cancer and three healthy cell lines, respectively, using an MTT-probing technique. Four pathogenic and three commensal aerobes were the microbes utilized to evaluate the antibacterial and microbiota compatibility, respectively, employing a broth microdilution methodology. The results indicated that the conjugate with the highest anticancer attribute and lowest cytotoxicity was SDC1, while SDC2 demonstrated the greatest antibacterial attribute with minimal microbiota toxicity. These findings projected the influence of a 3-position substitute of the coumarin backbone on the biological activities under research. From these, it is concluded that the chemical structures of SDC1 and SDC2 may represent potent anticancer cytocompatible and antibacterial microbiota-compatible scaffolds, respectively.
合成新型跳过二烯-3-卤代香豆素共轭物作为强效抗癌和抗菌生物相容性制剂
癌症和细菌感染是人类健康挥之不去的梦魇,如今,新型癌症和致病细菌的发展以及它们对处方药的抗药性加剧了这一问题。为了成为希望觉醒的一部分,这项研究旨在合成四种新型跳过二烯-3-卤代香豆素共轭物,代号为 SDC1-SDC4,并探索它们作为抗癌和抗菌替代品的生物安全功能。在合成的第一步,甲奎宁醇通过 H2SO4 催化的 Pechmann 反应与 2-卤乙酰乙酸乙酯反应,生成 3-卤-6-甲氧基-4-甲基香豆素。原位生成的 Vilsmeier-Haack 试剂将这些三官能化香豆素甲酰化,生成其 7-甲醛同系物。最后,通过改良的克莱森-施密特反应,将后者与 2,4-戊二酮缩合,制备出目标共轭物。通过分析傅立叶变换红外光谱(FTIR)、1H NMR、13C NMR 和 HRMS 谱图,确定了 SDC1-SDC4 的立体化学分子结构。另一方面,利用 MTT 检测技术研究了这些共轭物对 8 种癌症细胞株和 3 种健康细胞株的抗癌作用和细胞毒性。采用肉汤微稀释法,分别用四种病原菌和三种共生需氧菌来评估抗菌性和微生物群相容性。结果表明,抗癌特性最高、细胞毒性最低的共轭物是 SDC1,而 SDC2 的抗菌特性最强,微生物群毒性最小。这些发现说明了香豆素骨架的 3 位替代物对所研究生物活性的影响。由此得出结论,SDC1 和 SDC2 的化学结构可能分别代表了有效的抗癌细胞相容性支架和抗菌微生物群相容性支架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


