Supramolecular hybrid of 4-aminopyridinium Perchlorate: Synthesis, molecular structure characterization, vibrational spectroscopy, thermal analysis, and optical property evaluation
IF 2.5
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
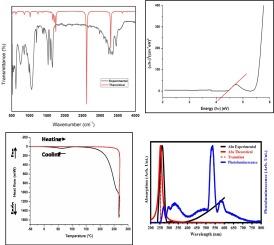
A hybrid material, 4-aminopyridinium perchlorate salt [C5H6N2][C5H7N2] ClO4, was synthesized at room temperature by slow evaporation and characterized by single-crystal X-ray, spectroscopic techniques (FT-IR, Raman, and UV–Visible), thermal studies (TGA and DSC). The crystallographic data obtained from single crystal X-ray analysis showed that the compound adopts the monoclinic system, space group P21/n, with the following parameters a = 10.8317(8) Å, b = 9.0529(5) Å, c = 13.7032(9) Å, β = 99.243(3) °, Z = 4 and V = 1326.27(15) Å3. The stability of the supramolecular structure was ensured by hydrogen bonding contacts with N-H…O lengths ranging between 2.749 and 2.940 Å. According to the results of Hirshfeld analysis, O…H interactions constitute the main intermolecular interaction contacts (46 %). The thermal decomposition of the precursors, studied by TGA and DSC analysis, indicates that the compound exhibits thermal stability up to 130 °C, beyond which it degrades. Infrared and Raman spectra were recorded at room temperature, confirming the existence of vibrational modes corresponding to organic and inorganic groups. Additionally, the optical properties of the synthesized compound exhibit UV–Visible absorption and interesting photoluminescence properties. The related HOMO-LUMO orbital energies were also highlighted using TDDFT calculations the B3LYP/6-31G (d,p) level of theory.
4-aminopyridinium Perchlorate 超分子杂化物:合成、分子结构表征、振动光谱、热分析和光学特性评估
通过单晶 X 射线、光谱技术(傅立叶变换红外光谱、拉曼光谱和紫外可见光谱)、热研究(TGA 和 DSC),在室温下用缓慢蒸发法合成了一种杂化材料--4-氨基吡啶高氯酸盐 [C5H6N2][C5H7N2] ClO4。单晶 X 射线分析获得的晶体学数据显示,该化合物采用单斜体系,空间群为 P21/n,参数如下:a = 10.8317(8) Å,b = 9.0529(5) Å,c = 13.7032(9) Å,β = 99.243(3) °,Z = 4,V = 1326.27(15) Å3。根据 Hirshfeld 分析结果,O...H 相互作用是分子间相互作用的主要接触(46%)。通过 TGA 和 DSC 分析对前体的热分解进行了研究,结果表明该化合物的热稳定性最高可达 130 °C,超过 130 °C则会降解。室温下记录的红外光谱和拉曼光谱证实了有机和无机基团振动模式的存在。此外,合成化合物的光学特性还表现出紫外-可见吸收和有趣的光致发光特性。利用 B3LYP/6-31G (d,p) 理论水平的 TDDFT 计算还突出了相关的 HOMO-LUMO 轨道能量。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


