A promising α-glucosidase and α-amylase inhibitors based on benzimidazole-oxadiazole hybrid analogues: Evidence based in vitro and in silico studies
IF 2.5
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
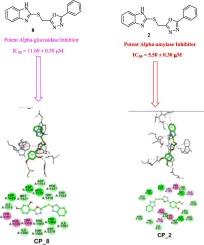
The present study reports the design and synthesis of new benzimidazole-oxadiazole compounds as potent inhibitors of α-glucosidase and α-amylase. The synthesized molecules were characterized through different techniques such as 1HNMR, 13CNMR, HREI-MS and evaluated for their in vitro inhibitory activities against these enzymes. Among the compounds screened, compound 8 demonstrated the highest inhibitory activity against both α-glucosidase (IC50 = 11.60 µM) and α-amylase (IC50 = 6.20 µM). Molecular docking analyses were conducted to investigate the binding modes and interactions of the active compounds within the enzyme active sites. The results demonstrate that several benzimidazole-oxadiazole hybrids exhibited potent inhibitory effects on both α-glucosidase and α-amylase, suggesting their promise as antidiabetic agents.
基于苯并咪唑-恶二唑杂化类似物的α-葡萄糖苷酶和α-淀粉酶抑制剂:基于证据的体外和硅学研究
本研究报告了作为 α-葡萄糖苷酶和 α-淀粉酶强效抑制剂的新型苯并咪唑-恶二唑化合物的设计与合成。通过 1HNMR、13CNMR、HREI-MS 等不同技术对合成的分子进行了表征,并评估了它们对这些酶的体外抑制活性。在筛选出的化合物中,化合物 8 对 α-葡萄糖苷酶(IC50 = 11.60 µM)和 α-淀粉酶(IC50 = 6.20 µM)的抑制活性最高。为研究活性化合物在酶活性位点内的结合模式和相互作用,进行了分子对接分析。结果表明,几种苯并咪唑-恶二唑杂交化合物对α-葡萄糖苷酶和α-淀粉酶都有很强的抑制作用,表明它们有望成为抗糖尿病药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


