A simple and highly efficient catalyst-free for the synthesis of dihydro pyrido[1, 2-a]quinoxaline derivatives by four component reactions and evaluation of their antibacterial activity
IF 2.5
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
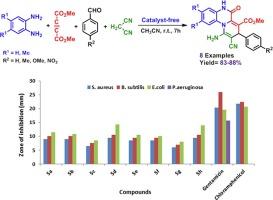
In the current work, a new series of methyl-10-amino-9-cyano-6-oxo-8-aryl-6,8-dihydro-5H-pyrido[1,2-a]quinoxaline-7-carboxylate derivatives were efficiently designed and prepared using a convenient route in excellent yields by four-component reactions among benzene-1,2-diamine, dimethyl acetylenedicarboxylate, various aromatic aldehydes and malononitrile. These reactions were carried out in acetonitrile at ambient temperature under catalyst-free conditions for 7 h. The structures of the new obtained compounds were confirmed by NMR, IR, EI-MS and elemental analysis. The antibacterial activity of the synthesized products was studied using bacterial strains: Staphylococcus aureus, Bacillus subtilis, Escherichia coli, and Pseudomonas aeruginosa. The results showed that all the synthesized compounds are effective against Staphylococcus aureus, Bacillus subtilis and Escherichia coli bacteria.
通过四组分反应合成二氢吡啶并[1, 2-a]喹喔啉衍生物的简单高效无催化剂方法及其抗菌活性评价
本研究采用简便的路线,通过苯-1,2-二胺、乙炔二甲酸二甲酯、多种芳香醛和丙二腈的四组分反应,有效地设计和制备了一系列新的甲基-10-氨基-9-氰基-6-氧代-8-芳基-6,8-二氢-5H-吡啶并[1,2-a]喹喔啉-7-羧酸酯衍生物,并获得了优异的产率。通过核磁共振、红外光谱、电离质谱和元素分析确认了新化合物的结构。利用细菌菌株对合成产物的抗菌活性进行了研究:金黄色葡萄球菌、枯草杆菌、大肠杆菌和绿脓杆菌。结果表明,所有合成化合物都对金黄色葡萄球菌、枯草杆菌和大肠杆菌有效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


