Enhancement of LiFePO4 cathodic material through incorporation of reduced graphene oxide via a simple two-step procedure
IF 4.3
3区 材料科学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
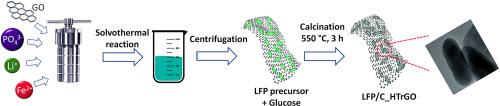
The aim of the present work is the improvement of LiFePO4 (LFP) as cathodic material for Lithium Batteries (LIBs) by controlled incorporation of graphene oxide (GO) and its subsequent reduction during LFP synthesis. The electrochemical performance of the samples is analysed after reduction treatment of GO in different steps of the synthesis. The purpose of the reduction is to provide LFP particles with higher conductivity through a uniform conductive carbon coating that facilitates the migration of Li+ ions in the cathode for charge-discharge process. These electrochemical measurements revealed an initial discharge capacity of 70.19 mAh g−1 for LFP without GO, 141.3 mAh g−1 and 154.3 mAh g−1 with GO added in heat-treatment and solvothermal step, at 0.5C rate, respectively. Additionally, the cycling stability of the samples with GO improves the corresponding one obtained for pristine LFP. At 0.5C rate cycle-life, the LFP with GO combined with raw materials during solvothermal synthesis, attained capacity retention of 99 % after 100 cycles, decreasing to values of 88 % and 87 % for LFP with GO incorporated just before thermal treatment and LFP without GO. These results indicate that the LFP with GO incorporated initially in the synthesis improves rate capability of the material, reaching reversible capacity of 158.5 mAh g−1 at 0.1C, 154.4 mAh g−1 at 0.2C, 151.3 mAh g−1 at 0.5C, 122.7 mAh g−1 at 1C and 112.6 mAh g−1 at 2C. The improvement in electrochemical behaviour can be attributed to the optimization of the synthesis process that provides a well-crosslinked interior of the composite and, therefore, a stable conductive network which has an associated higher diffusion coefficient.
通过简单的两步程序加入还原氧化石墨烯,增强 LiFePO4 阴极材料的性能
本研究的目的是通过控制氧化石墨烯(GO)的加入以及随后在 LFP 合成过程中对其进行还原处理,改进作为锂电池(LIB)阴极材料的 LiFePO4(LFP)。在合成的不同步骤中对 GO 进行还原处理后,对样品的电化学性能进行了分析。还原处理的目的是通过均匀的导电碳涂层使 LFP 颗粒具有更高的导电性,从而促进 Li+ 离子在阴极中的迁移,实现充放电过程。这些电化学测量结果表明,在 0.5C 速率下,不添加 GO 的 LFP 初始放电容量为 70.19 mAh g-1,添加 GO 的 LFP 初始放电容量为 141.3 mAh g-1 和 154.3 mAh g-1。此外,添加了 GO 的样品的循环稳定性比原始 LFP 的相应稳定性更高。在 0.5 摄氏度的循环寿命下,溶解热合成过程中添加了 GO 的 LFP 与原材料结合,在 100 次循环后的容量保持率达到 99%,而在热处理前添加了 GO 的 LFP 和未添加 GO 的 LFP 的容量保持率分别为 88% 和 87%。这些结果表明,在合成初期加入 GO 的 LFP 提高了材料的速率能力,其可逆容量在 0.1C 时达到 158.5 mAh g-1,0.2C 时达到 154.4 mAh g-1,0.5C 时达到 151.3 mAh g-1,1C 时达到 122.7 mAh g-1,2C 时达到 112.6 mAh g-1。电化学性能的改善可归功于合成工艺的优化,它使复合材料的内部交联良好,从而形成了稳定的导电网络,并相应提高了扩散系数。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
2.50%
发文量
605
审稿时长
40 days
期刊介绍:
The Journal of Physics and Chemistry of Solids is a well-established international medium for publication of archival research in condensed matter and materials sciences. Areas of interest broadly include experimental and theoretical research on electronic, magnetic, spectroscopic and structural properties as well as the statistical mechanics and thermodynamics of materials. The focus is on gaining physical and chemical insight into the properties and potential applications of condensed matter systems.
Within the broad scope of the journal, beyond regular contributions, the editors have identified submissions in the following areas of physics and chemistry of solids to be of special current interest to the journal:
Low-dimensional systems
Exotic states of quantum electron matter including topological phases
Energy conversion and storage
Interfaces, nanoparticles and catalysts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


