The mechanism of hydrogen generation from H2O splitting of Ben (n = 14–17) clusters based on density functional theory
IF 4.3
3区 材料科学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The conventional method of producing hydrogen does not promote sustainable development and gravely damages the environment. Nevertheless, this paper studies investigate the connection between the magic numbers (4, 10, 17) and the reactions of Ben (n = 14–17) clusters splitting H2O to produce H2 as well as the reaction mechanism, solving the environmental pollution problem. This experiment reveals the mechanism of hydrogen generation from H2O splitting of Ben (n = 14–17) clusters using the PBE0 functional and the def2-TZVP basis, which is based on density functional theory. We have plotted the energy gap diagrams, interaction region indicator diagrams, and density of state diagrams of Ben (n = 14–17) clusters in order to explore the intermolecular interactions and energy changes that occur during the adsorption and desorption of water molecule and clusters. The findings demonstrate that the reactions of Ben (n = 14–17) clusters splitting H2O to produce H2 is releasing energy. The Ben (n = 14–17) clusters have the best hydrogen evolution efficiency when the number of atoms is near the magic number.
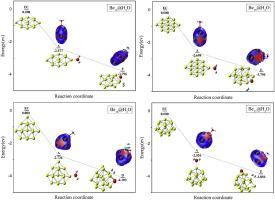
基于密度泛函理论的 Ben(n = 14-17)团簇 H2O 裂解产生氢的机理
传统的制氢方法不利于可持续发展,而且严重破坏环境。然而,本文研究了神奇数字(4、10、17)与本(n = 14-17)簇分裂 H2O 产生氢气的反应之间的联系以及反应机理,解决了环境污染问题。本实验利用基于密度泛函理论的 PBE0 函数和 def2-TZVP 基础,揭示了 Ben (n = 14-17) 团簇分裂 H2O 产生氢气的机理。我们绘制了 Ben (n = 14-17) 团簇的能隙图、相互作用区域指示图和状态密度图,以探索水分子和团簇在吸附和解吸过程中发生的分子间相互作用和能量变化。研究结果表明,Ben(n = 14-17)簇分裂 H2O 生成 H2 的反应正在释放能量。当原子数接近魔数时,Ben(n = 14-17)团簇的氢进化效率最高。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
2.50%
发文量
605
审稿时长
40 days
期刊介绍:
The Journal of Physics and Chemistry of Solids is a well-established international medium for publication of archival research in condensed matter and materials sciences. Areas of interest broadly include experimental and theoretical research on electronic, magnetic, spectroscopic and structural properties as well as the statistical mechanics and thermodynamics of materials. The focus is on gaining physical and chemical insight into the properties and potential applications of condensed matter systems.
Within the broad scope of the journal, beyond regular contributions, the editors have identified submissions in the following areas of physics and chemistry of solids to be of special current interest to the journal:
Low-dimensional systems
Exotic states of quantum electron matter including topological phases
Energy conversion and storage
Interfaces, nanoparticles and catalysts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


