Photocatalytic reduction of nitroarene derivatives by impressive visible-light active Co3O4-QDs/Mn3O4 nanocomposite
IF 4.3
3区 材料科学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
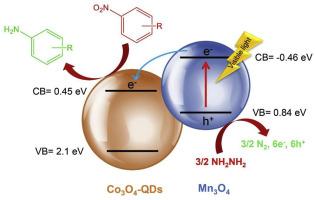
A visible light active Co3O4-quantum dots (QDs)/Mn3O4 nanocomposite was successfully synthesized by a straightforward precipitation approach. Notably, the formation of Mn3O4 nanosheets in composite structure was evidenced by different physicochemical techniques especially transmission electron microscopy (TEM), energy dispersive X-ray (EDX) and N2 sorption analyses, which indicated the uniform distribution of Co3O4-QDs with small size of around 5 nm on the surface of Mn3O4 nanosheets along with the enhancement of specific surface area for final composite. Also, the persistence of Co3O4-QDs during nanocomposite synthesis was analyzed by X-ray diffraction (XRD), FT-IR and TEM techniques. The diffuse reflectance UV–Vis and electrochemical impedance spectroscopies revealed the reduction of band gap energy and enhanced charge separation efficiency for Co3O4-QDs/Mn3O4 nanocomposite, respectively, which consequently led to improved photocatalytic response in comparison with bare Co3O4-QDs and Mn3O4 nanosheets. The as-prepared nanocomposite exhibited excellent photocatalytic activity in the reduction of a wide range of substituted nitroarenes with the yields of higher than 80 % relative to the corresponding arylamines, using N2H4·H2O as the hydrogen source and ethanol as green solvent at 25 °C and ambient pressure under visible light illumination. The synergistic effect could improve the production of active hydrogen atoms, therefore, the reasons for this transformation over the prepared nanocomposite under benign conditions are fully discussed. Furthermore, the nanocomposite could be easily recycled without decay in photocatalytic activity for at least five successive cycles.
令人印象深刻的可见光活性 Co3O4-QDs/Mn3O4 纳米复合材料对硝基炔衍生物的光催化还原作用
通过直接沉淀法成功合成了一种可见光活性 Co3O4-量子点(QDs)/Mn3O4 纳米复合材料。值得注意的是,不同的理化技术,特别是透射电子显微镜(TEM)、能量色散 X 射线(EDX)和 N2 吸附分析,证明了复合结构中 Mn3O4 纳米片的形成,这表明小尺寸的 Co3O4-QDs (约 5 纳米)均匀分布在 Mn3O4 纳米片表面,并提高了最终复合材料的比表面积。此外,还通过 X 射线衍射 (XRD)、傅立叶变换红外光谱 (FT-IR) 和 TEM 技术分析了 Co3O4-QDs 在纳米复合材料合成过程中的持久性。漫反射紫外可见光谱和电化学阻抗光谱显示,Co3O4-QDs/Mn3O4 纳米复合材料分别降低了带隙能和提高了电荷分离效率,因此与裸 Co3O4-QDs 和 Mn3O4 纳米片相比,光催化响应有所改善。以 N2H4-H2O 为氢源,乙醇为绿色溶剂,在 25 °C、环境压力和可见光光照条件下,制备的纳米复合材料在还原多种取代的硝基烯烃时表现出优异的光催化活性,相对于相应的芳基胺,产率超过 80%。这种协同效应可以提高活性氢原子的产生,因此,我们充分讨论了在良性条件下制备的纳米复合材料发生这种转变的原因。此外,这种纳米复合材料可以很容易地循环使用,至少连续循环五次,光催化活性都不会衰减。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
2.50%
发文量
605
审稿时长
40 days
期刊介绍:
The Journal of Physics and Chemistry of Solids is a well-established international medium for publication of archival research in condensed matter and materials sciences. Areas of interest broadly include experimental and theoretical research on electronic, magnetic, spectroscopic and structural properties as well as the statistical mechanics and thermodynamics of materials. The focus is on gaining physical and chemical insight into the properties and potential applications of condensed matter systems.
Within the broad scope of the journal, beyond regular contributions, the editors have identified submissions in the following areas of physics and chemistry of solids to be of special current interest to the journal:
Low-dimensional systems
Exotic states of quantum electron matter including topological phases
Energy conversion and storage
Interfaces, nanoparticles and catalysts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


