Synthesis of various types of green biosorbents materials for removals of sulphates from contaminated water for better aquatic environments
引用次数: 0
Abstract
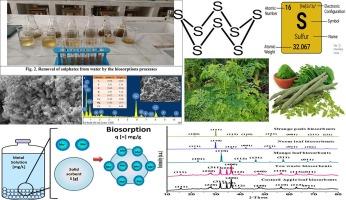
Human and industrial activities pollute water resources with sulphur metal, endangering human health and ecosystems. Chemical precipitation and membrane filtration are expensive when treating large amounts of water, inefficient at low metal concentrations, and produce large amounts of toxic sludge and other products that must be disposed of waste water bio-sorption is eco-friendly and alternative. These methods are cheaper, more accessible, and reusable than conventional ones. This study investigates the bio-sorption of sulphur from contaminated water using neem leaf, custard apple leaf, mango tree leaf, orange peels, and banana peels biological waste materials. This work achieves 100 % removal efficiency. Biosorbents remove sulphates from contaminated water: custard leaves 100 % at 4gm, orange peels 40 % at 5gm, tea waste 50 % at 4gm, neem leaves 70 % at 5gm, and mango leaves 75 % at 5gm. The performances of biosorbents for removals of sulphates from water as follows: Custard leaves waste > Mango leaves > Neem leaves > Tea trash > Orange peels the sulphate reduction by biosorbents. The best biosorption occurred at basic pH 6.5, 3.7gm dosage, 90 min contact duration, 30 °C temperature, and 120 rpm agitation speed. The effects of contact time, agitation speed, adsorbent dosage, pH, and temperature are also examined. Before usage, biosorbents materials can be physically and chemically measured the changes. Regenerating and reusing bio-sorbent after sulphates removals makes the method cost-effective for removals of pollutants from water.
合成各种类型的绿色生物吸附剂材料,用于去除受污染水体中的硫酸盐,改善水生环境
人类活动和工业活动使水资源受到金属硫的污染,危及人类健康和生态系统。化学沉淀法和膜过滤法在处理大量水时成本高昂,在处理低浓度金属时效率低,而且会产生大量有毒污泥和其他产品,必须对废水进行处理。与传统方法相比,这些方法更便宜、更易获得、可重复使用。本研究利用楝树叶、吉士苹果叶、芒果树叶、橘子皮和香蕉皮等生物废料对受污染水中的硫进行生物吸附。这项工作实现了 100% 的去除效率。生物吸附剂从受污染的水中去除硫酸盐的情况如下:吉士苹果叶 4gm 时去除率为 100%,橘子皮 5gm 时去除率为 40%,茶叶废料 4gm 时去除率为 50%,楝树叶 5gm 时去除率为 70%,芒果树叶 5gm 时去除率为 75%。生物吸附剂去除水中硫酸盐的性能如下:吉士叶垃圾、芒果叶、印度楝树叶、茶叶垃圾和橘子皮的生物吸附剂减少了硫酸盐的含量。在 pH 值为 6.5、用量为 3.7 毫克、接触时间为 90 分钟、温度为 30 °C、搅拌速度为 120 转/分的条件下,生物吸附效果最好。此外,还研究了接触时间、搅拌速度、吸附剂用量、pH 值和温度的影响。在使用之前,可以对生物吸附剂材料的物理和化学变化进行测量。生物吸附剂在去除硫酸盐后可以再生和重复使用,这使得该方法在去除水中污染物方面具有成本效益。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


