Sophora davidii Hance leaves total alkaloids (SDLTAs) alleviate asthma through inhibiting airway inflammation and regulating TLR4/MyD88/c-Jun pathway based on systematic pharmacology and molecular docking
IF 3.8
2区 农林科学
Q2 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
Asthma is a complex heterogeneous and inflammatory disease with an increasing incidence worldwide. Here, UHPLC-Q-Exactive-MSE analysis revealed that 21 alkaloids and 1 coumarin compound (Scopoletin) were identified in SDLTAs. In mouse model, SDLTAs treatment reduced the airway pathological damage, the levels of IL-4, IL-5, IgE, MUC5AC, and splenic index. In cell model, SDLTAs treatment significantly decreased IL-6, IL-8, MUC5AC, and mucus secretion induced by LPS. Network pharmacology studies revealed that the main active ingredients of SDLTAs, including oxysophoridine, oxymatrine, scopoletin and sophoridine, target TLR4, DPP4, IL6 and TNF, as well as regulate IL-17 signaling pathway, TNF signaling pathway, and toll-like receptor signaling pathway, leading to relief of asthma on an inflammatory basis. Docking results showed that the binding energy of the main active ingredients of SDLTAs to TLR4 and MyD88 varied from −5.38 kcal/mol to −4.01 kcal/mol. Further, SDLTAs down-regulated the expression of TLR4, MyD88 and p-c-Jun.
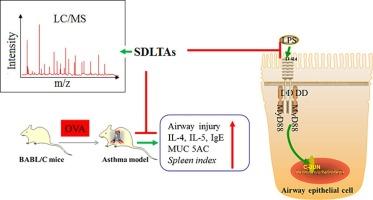
基于系统药理学和分子对接的槐叶总生物碱(SDLTAs)通过抑制气道炎症和调节 TLR4/MyD88/c-Jun 通路缓解哮喘
哮喘是一种复杂的异质性炎症性疾病,在全世界的发病率越来越高。本研究通过超高效液相色谱-Q-Exactive-MSE分析发现,SDLTAs中含有21种生物碱和1种香豆素化合物(Scopoletin)。在小鼠模型中,SDLTAs能减轻气道病理损伤,降低IL-4、IL-5、IgE、MUC5AC水平和脾脏指数。在细胞模型中,SDLTAs能显著降低LPS诱导的IL-6、IL-8、MUC5AC和粘液分泌。网络药理学研究表明,SDLTAs 的主要有效成分,包括oxysophoridine、oxymatrine、scopoletin 和 sophoridine,可靶向 TLR4、DPP4、IL6 和 TNF,并调节 IL-17 信号通路、TNF 信号通路和 toll 样受体信号通路,从而在炎症基础上缓解哮喘。Docking结果显示,SDLTAs的主要活性成分与TLR4和MyD88的结合能从-5.38 kcal/mol到-4.01 kcal/mol不等。此外,SDLTAs还能下调TLR4、MyD88和p-c-Jun的表达。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Functional Foods
FOOD SCIENCE & TECHNOLOGY-
CiteScore
9.60
自引率
1.80%
发文量
428
审稿时长
76 days
期刊介绍:
Journal of Functional Foods continues with the same aims and scope, editorial team, submission system and rigorous peer review. We give authors the possibility to publish their top-quality papers in a well-established leading journal in the food and nutrition fields. The Journal will keep its rigorous criteria to screen high impact research addressing relevant scientific topics and performed by sound methodologies.
The Journal of Functional Foods aims to bring together the results of fundamental and applied research into healthy foods and biologically active food ingredients.
The Journal is centered in the specific area at the boundaries among food technology, nutrition and health welcoming papers having a good interdisciplinary approach. The Journal will cover the fields of plant bioactives; dietary fibre, probiotics; functional lipids; bioactive peptides; vitamins, minerals and botanicals and other dietary supplements. Nutritional and technological aspects related to the development of functional foods and beverages are of core interest to the journal. Experimental works dealing with food digestion, bioavailability of food bioactives and on the mechanisms by which foods and their components are able to modulate physiological parameters connected with disease prevention are of particular interest as well as those dealing with personalized nutrition and nutritional needs in pathological subjects.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


