Diastereoselective Stereo-Divergent Synthesis of Spiroindolines via Ligand-Controlled Silver(I)-Catalyzed Asymmetric [3 + 2] cycloadditions
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A series of spirocyclic pyrrolidine derivatives with quaternary stereocenters was constructed via asymmetric [3 + 2] cycloaddition reactions between imino esters and 2-oxindole. By ingenious use of ligands on the silver catalyst allows stereoselectivity to either endo-isomers (ee up to 98 %, yields up to 96 %) with (R, Rp)-Ph-Phosferrox or exo-isomers (ee up to 99 %, yields up to 87 %) with (R)-BINAP. This work is notable for its high yields, broad substrate adaptability and excellent enantioselectivity.
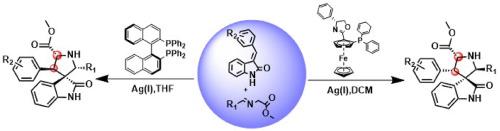
通过配体控制的银(I)催化不对称[3 + 2]环加成反应非对映选择性立体发散合成螺吲哚类化合物
通过亚氨基酯和 2-oxindole 之间的不对称 [3 + 2] 环加成反应,制备了一系列具有四元立体中心的螺环吡咯烷衍生物。通过在银催化剂上巧妙地使用配体,可以通过 (R, Rp)-Ph-Phosferrox 实现内向异构体(ee 高达 98%,产率高达 96%)的立体选择性,或通过 (R)-BINAP 实现外向异构体(ee 高达 99%,产率高达 87%)的立体选择性。这项工作的显著特点是产率高、底物适应性广和对映体选择性好。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


