Ru-catalyzed asymmetric transfer hydrogenation of racemic β-keto γ-lactams via dynamic kinetic resolution
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The enantioselective transfer hydrogenation of racemic β-keto γ-lactams via dynamic kinetic resolution using a chiral Ru(II) catalyst has been developed for the synthesis of optically active β-hydroxyl lactams with excellent conversion (up to 99 %), high diastereomeric ratio (dr 93:07) and enantiomeric selectivity (89 % ee). The reaction proceeded by using HCO2H/Et3N as hydrogen donor and features mild, additive free reaction conditions, fast crystallization, broad substrate scope, and an operationally simpler setup than that for molecular hydrogenation.
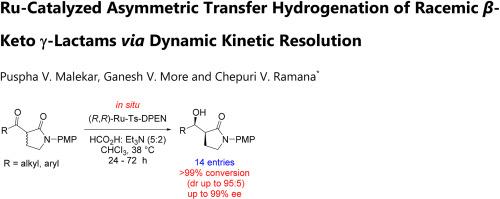
通过动态动力学解析 Ru 催化外消旋 β-酮 γ-内酰胺的不对称转移加氢反应
利用手性 Ru(II) 催化剂,通过动态动力学解析,开发了外消旋β-酮γ-内酰胺的对映选择性转移加氢反应,用于合成光学活性β-羟基内酰胺,该反应具有优异的转化率(高达 99 %)、高非对映异构比(dr 93:07)和对映选择性(89 % ee)。该反应以 HCO2H/Et3N 为氢供体,反应条件温和,不含添加剂,结晶速度快,底物范围广,操作设置比分子氢化简单。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


