An acid-mediated synthesis of substituted naphthalenes from ortho-alkynyl tertiary benzylic alcohols
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We present an efficient, acid-mediated cascade strategy for the construction of 1,3- and 1,2,3-substituted naphthalenes from readily accessible starting materials. This method employs ortho-alkynyl or arynyl tertiary benzylic alcohols as the substrates, which undergo a Lewis acid-triggered dehydration to form ortho-(alkynyl)-styrene intermediates. These intermediates subsequently undergo intramolecular cycloaromatization, resulting in the formation of substituted naphthalenes in a one-pot reaction. A significant advantage of this approach is its ability to proceed under sustainable and neat reaction conditions using ZnCl₂ as the sole Lewis acid, highlighting the simplicity and practicality of the process. Additionally, the strategy exhibits a broad substrate scope, efficiently yielding the desired naphthalene derivatives with practical and consistent yields. This method not only simplifies the synthetic pathway but also aligns with green chemistry principles by minimizing the use of solvents and reagents.
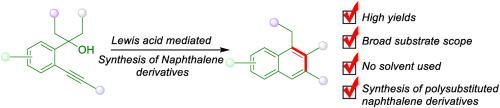
酸介导的邻炔基三级苄醇合成取代萘的方法
我们提出了一种以酸为介质的高效级联策略,用于从容易获得的起始材料中构建 1,3- 和 1,2,3- 取代的萘。这种方法采用正炔基或芳基叔苄醇作为底物,经过路易斯酸引发的脱水作用形成正(炔基)苯乙烯中间体。这些中间体随后发生分子内环芳香化反应,从而在一锅反应中形成取代萘。这种方法的一个显著优点是能够在可持续和纯净的反应条件下进行,使用 ZnCl₂作为唯一的路易斯酸,突出了该过程的简便性和实用性。此外,该策略还具有广泛的底物范围,能高效地生成所需的萘衍生物,而且产率实用、稳定。这种方法不仅简化了合成途径,而且通过尽量减少溶剂和试剂的使用,符合绿色化学原则。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


