I2-promoted direct C–H arylselenylation of pyrrolo[1,2-a]quinoxalines
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
An I2-promoted C–H arylselenylation of pyrrolo[1,2-a]quinoxalines with diaryl diselenides is developed, providing an efficient route to a series of 1 (or 3)-arylselenylated and/or 1,3-diaryl diselenylated pyrrolo[1,2-a]quinoxalines. The methodology is characterised by a wide range of substrates, good functional group tolerance and gram-level synthesis. Further transformations of the products to form structurally diverse pyrrolo[1,2-a]quinoxalines were successfully achieved. Similarly, I2-promoted C–H sulfenylation of pyrrolo[1,2-a]quinoxaline with 1,2-diphenyldisulfane were investigated. We believe that these novel pyrrolo[1,2-a]quinoxaline compounds will have promising applications in pharmaceutical synthesis.
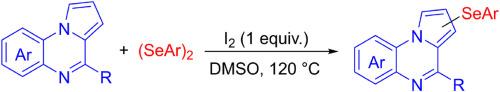
I2 促进的吡咯并[1,2-a]喹喔啉的直接 C-H 芳基硒化反应
本研究开发了一种 I2 促进的吡咯并[1,2-a]喹喔啉与二芳基二硒化物的 C-H 芳基硒化反应,为获得一系列 1 (或 3) -芳基硒化和/或 1,3 -二芳基二硒化的吡咯并[1,2-a]喹喔啉提供了一条有效途径。该方法具有底物范围广、官能团耐受性好和克级合成等特点。成功实现了产品的进一步转化,形成了结构多样的吡咯并[1,2-a]喹喔啉类化合物。同样,我们还研究了 I2 促进的吡咯并[1,2-a]喹喔啉与 1,2-二苯基二硫醚的 C-H 磺化反应。我们相信,这些新型吡咯并[1,2-a]喹喔啉化合物将在药物合成中具有广阔的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


