Regulation mechanism of exogenous nitric oxide on phenanthrene uptake by ryegrass roots
IF 6.1
2区 生物学
Q1 PLANT SCIENCES
引用次数: 0
Abstract
Polycyclic aromatic hydrocarbons (PAHs) constitute a category of persistent organic contaminants that possess the potential to induce carcinogenic, teratogenic, and mutagenic consequences. Our previous findings have revealed that plant roots actively take up PAHs through co-transport with protons, and auxin can promote PAHs uptake by wheat roots. It remains unclear whether nitric oxide (NO), a signaling molecule involved in numerous physiological processes in plants and downstream of auxin, can affect PAHs uptake by plant roots. In our study, 50 μmol/L sodium nitroprusside (SNP) significantly enhanced phenanthrene uptake after 4 h of exposure. After the addition of SNP (50 μmol/L), the H+ flux on root surface increased, and H+-ATPase activity was activated, indicating that exogenous NO promotes phenanthrene uptake by plant roots via activating H+-ATPase. By studying the effects of 50 μmol/L cyclic guanosine monophosphate (cGMP), 5 mmol/L Ca2+, and 50 μmol/L adenosine monophosphate (AMP) on phenanthrene uptake by ryegrass roots and measuring root calcium-dependent protein kinases (CDPK) activity, we demonstrated that exogenous NO promotes phenanthrene uptake through the signaling pathway of NO, cGMP, Ca2+, CDPK, 14-3-3 protein and H+-ATPase. The results contribute significant insights into elucidating the underlying mechanisms of NO modulating PAHs absorption by plant roots, thereby offering crucial strategies for advancing food safety measures and enhancing the phytoremediation potential of soils and waters contaminated with PAHs.
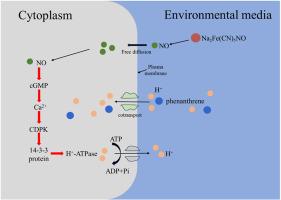
外源一氧化氮对黑麦草根系吸收菲的调节机制
多环芳烃(PAHs)是一类具有致癌、致畸和致突变潜力的持久性有机污染物。我们之前的研究结果表明,植物根系通过与质子的共转运主动吸收多环芳烃,而辅酶能促进小麦根系吸收多环芳烃。一氧化氮(NO)是参与植物许多生理过程的信号分子,也是辅助素的下游,它是否会影响植物根系对 PAHs 的吸收,目前仍不清楚。在我们的研究中,50 μmol/L 硝普钠(SNP)能显著提高暴露 4 小时后菲的吸收。加入 SNP(50 μmol/L)后,根表面的 H+ 通量增加,H+-ATP 酶活性被激活,表明外源 NO 通过激活 H+-ATP 酶促进植物根系对菲的吸收。通过研究 50 μmol/L 环鸟苷单磷酸(cGMP)、5 mmol/L Ca2+和 50 μmol/L 腺苷单磷酸(AMP)对黑麦草根吸收菲的影响,并测定根的钙依赖性蛋白激酶(CDPK)活性、结果表明,外源 NO 通过 NO、cGMP、Ca2+、CDPK、14-3-3 蛋白和 H+-ATP 酶的信号途径促进菲的吸收。这些结果为阐明氮氧化物调节植物根系吸收多环芳烃的内在机制提供了重要见解,从而为推进食品安全措施和提高受多环芳烃污染的土壤和水体的植物修复潜力提供了重要策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.10
自引率
3.10%
发文量
410
审稿时长
33 days
期刊介绍:
Plant Physiology and Biochemistry publishes original theoretical, experimental and technical contributions in the various fields of plant physiology (biochemistry, physiology, structure, genetics, plant-microbe interactions, etc.) at diverse levels of integration (molecular, subcellular, cellular, organ, whole plant, environmental). Opinions expressed in the journal are the sole responsibility of the authors and publication does not imply the editors'' agreement.
Manuscripts describing molecular-genetic and/or gene expression data that are not integrated with biochemical analysis and/or actual measurements of plant physiological processes are not suitable for PPB. Also "Omics" studies (transcriptomics, proteomics, metabolomics, etc.) reporting descriptive analysis without an element of functional validation assays, will not be considered. Similarly, applied agronomic or phytochemical studies that generate no new, fundamental insights in plant physiological and/or biochemical processes are not suitable for publication in PPB.
Plant Physiology and Biochemistry publishes several types of articles: Reviews, Papers and Short Papers. Articles for Reviews are either invited by the editor or proposed by the authors for the editor''s prior agreement. Reviews should not exceed 40 typewritten pages and Short Papers no more than approximately 8 typewritten pages. The fundamental character of Plant Physiology and Biochemistry remains that of a journal for original results.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


