1,2,3-Triazole nilotinib analogues: Synthesis and Cytotoxic activity
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Using the CuAAC reaction as a key step, a novel series of Nilotinib analogues was prepared by replacing the imidazole ring in the Nilotinib molecule with 1,2,3-triazole moiety via a divergent synthetic approach. The synthesized compounds were tested for cytotoxic activity against a series of tumor cell lines which included U251, PC-3, K562, HCT-15, MCF-7 and SKLU. Inhibition growth up to 85.6 % was observed for some compounds.
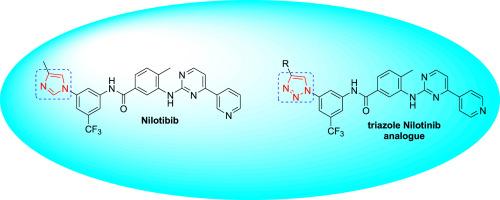
1,2,3-三唑尼洛替尼类似物:合成与细胞毒性活性
以 CuAAC 反应为关键步骤,通过发散合成方法将尼洛替尼分子中的咪唑环替换为 1,2,3 三唑分子,制备出一系列新型尼洛替尼类似物。合成的化合物对一系列肿瘤细胞系(包括 U251、PC-3、K562、HCT-15、MCF-7 和 SKLU)进行了细胞毒活性测试。结果表明,一些化合物对肿瘤细胞生长的抑制率高达 85.6%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


