Mechanistic insights of surface OH* modulation on methanol production with CO2 hydrogenation by iron-based catalyst
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The conversion of CO2 into high-value-added chemicals via the Fischer-Tropsch Synthesis (FTS) reaction has gathered a lot of attention. The surface oxygenation environment is a significant factor affecting the catalyst performance. In this work, spin-polarized density-functional theory calculations have been used to investigate the adsorption and reactions of CO2 and H to generate CH4 and CH3OH on Fe5C2(510) surfaces with varying OH* coverage. On the pure Fe5C2(510) surface, CO2 preferentially dissociates via direct dissociation, and the major C1 species generated is CH4. At low OH* coverage, the preferential pathway for CO2 dissociation changes from direct dissociation to the H-assisted route by the formation of COOH*. The major C1 product of the reaction in this state is transferred to CH3OH. In addition, CO2 hydrogenation reactions are facilitated by the OH* species. At high OH coverage, CO2 preferentially dissociates through the HCOO* intermediates. However, it appears that the CO2 hydrogenation reaction activity is suppressed. The results demonstrate that maintaining the surface environment with OH* and H* could be an indispensable measure to obtain the target product in the iron-based CO2 Fischer-Tropsch Synthesis system.
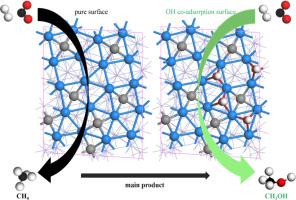
铁基催化剂表面 OH* 调制对二氧化碳加氢制甲醇的机理启示
通过费托合成(FTS)反应将二氧化碳转化为高附加值化学品已引起广泛关注。表面含氧环境是影响催化剂性能的一个重要因素。在这项研究中,我们利用自旋极化密度泛函理论计算研究了 CO2 和 H 在不同 OH* 覆盖率的 Fe5C2(510) 表面的吸附和反应,以生成 CH4 和 CH3OH。在纯净的 Fe5C2(510) 表面上,CO2 优先通过直接解离,生成的主要 C1 物种是 CH4。当 OH* 覆盖率较低时,CO2 的优先解离途径由直接解离变为 H 辅助途径,形成 COOH*。在这种状态下,反应的主要 C1 产物转化为 CH3OH。此外,OH* 物种也促进了 CO2 加氢反应。在 OH 覆盖率较高的情况下,CO2 会优先通过 HCOO* 中间产物解离。不过,CO2 加氢反应的活性似乎受到了抑制。结果表明,在铁基 CO2 费托合成系统中,保持表面环境中的 OH* 和 H* 是获得目标产物不可或缺的措施。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


