Laser-engraved defects in TiO2 support: Enhancing reducibility and redox capability of Pt/TiO2 catalyst for reactive and selective hydrogenation
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Titanium dioxide (TiO2) has been studied as catalyst or catalyst support in catalysis. Its synthesis or modification approach controls the structural, optical, and electronic properties. Here we applied laser engraving to the anatase TiO2 and studied the consequent changes in its structure and property as well as the properties of TiO2 supported platinum (i.e., Pt/TiO2) catalyst. The laser engraving enlarged the particle size, formed rutile phase and created defects (i.e., oxygen vacancy (Ov) and Ti3+) in anatase TiO2. This induced band gap change and enhanced visible light absorption. The defects created by laser engraving are stable and more reducible than those existed in the pristine TiO2. The defective TiO2 is structurally stable and has great redox properties. The metal-support interaction in the Pt/defective TiO2 catalyst is stronger than that of the pristine Pt/TiO2 catalyst, which enabled higher reactivity and selectivity in hydrogenation of 3-nitrostyrene and furfuryl alcohol. Laser-engraved TiO2 has been rarely studied for thermal catalysis. This work provides basic understanding of material properties and catalysis application of laser-engraved catalyst supports and catalysts in field of thermal catalysis.
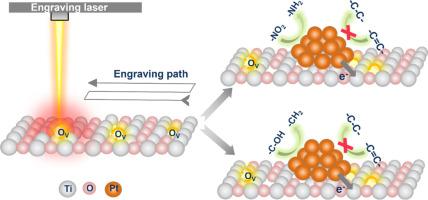
二氧化钛载体中的激光刻蚀缺陷:提高 Pt/TiO2 催化剂的还原性和氧化还原能力,用于反应性和选择性氢化
二氧化钛(TiO2)作为催化剂或催化剂载体在催化领域一直备受研究。其合成或改性方法可控制其结构、光学和电子特性。在此,我们对锐钛矿二氧化钛进行了激光雕刻,并研究了其结构和性质的相应变化,以及二氧化钛支撑的铂(即铂/二氧化钛)催化剂的性质。激光雕刻扩大了锐钛型二氧化钛的粒度,形成了金红石相,并在锐钛型二氧化钛中产生了缺陷(即氧空位(Ov)和 Ti3+)。这引起了带隙变化,增强了对可见光的吸收。与原始二氧化钛中存在的缺陷相比,激光雕刻产生的缺陷更稳定、更易还原。有缺陷的二氧化钛结构稳定,具有很好的氧化还原特性。与原始铂/二氧化钛催化剂相比,铂/缺陷二氧化钛催化剂中的金属-支撑相互作用更强,因此在 3-硝基苯乙烯和糠醇的氢化反应中具有更高的反应活性和选择性。激光刻蚀 TiO2 在热催化方面的研究很少。这项研究为激光雕刻催化剂载体和催化剂在热催化领域的材料特性和催化应用提供了基本认识。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


