Engineering an alcohol dehydrogenase from Gluconobacter oxydans for improved production of a bulky Ezetimibe intermediate
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
(4S)-3-[(5S)-5-(4-fluorophenyl)-5‑hydroxy-valyl]-4-phenyl-1,3-oxazacyclopentane-2-one ((S)-Fop alcohol) is a key chiral intermediate for the synthesis of ezetimibe, and could be synthesized via asymmetric reduction of (S)-4-phenyl-3-[5-(4-fluorophenyl)-5-oxopentanoyl]-2-oxazolidione (Fop dione). However, discovering and engineering of ketoreductases toward bulky-bulky (diaryl) ketones is still challenging. Previously, we identified an alcohol dehydrogenase Gox0525 from Gluconobacter oxydans DSM2343 which possessed strict diastereoselectivity (d.e. value > 99%) but low activity toward Fop dione. In this study, a semi-rational design based on the focused rational iterative site-specific mutagenesis (FRISM) based on site-directed saturation mutagenesis was performed to improve the catalytic efficiency of Gox0525. The variant M4 (Y92G/P93M/Y94P/L151V) shows a 64-fold enhanced catalytic efficiency (Kcat/Km) and 47-fold in specific activity compared with the wild type Gox0525. Engineered Escherichia coli cells co-expressing the variant M4 and glucose dehydrogenase from Bacillus subtilis (BsGDH) for NADPH regeneration were employed as biocatalysts for gram-scale reaction of Fop dione. As a result,95 mM (33.76 g/L) Fop dione was completely transformed within 4 h, affording (S)-Fop alcohol with > 99% d.e. value, the yield of 96%, and the space-time yield of 195.6 g/L/d.
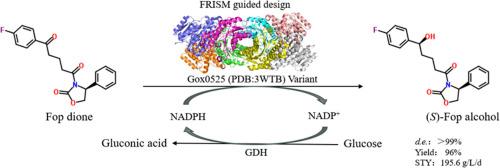
从氧葡萄糖杆菌中改造出一种醇脱氢酶,以改进大体积依折麦布中间体的生产
(4S)-3-[(5S)-5-(4-氟苯基)-5-羟基-缬氨酰]-4-苯基-1,3-恶唑杂环戊烷-2-酮((S)-Fop 醇)是合成依折麦布的关键手性中间体,可通过 (S)-4-phenyl-3-[5-(4-fluorophenyl)-5-oxopentanoyl]-2-oxazolidione (Fop dione) 的不对称还原合成。然而,发现和工程化酮还原酶来处理笨重的(二芳基)酮类化合物仍然具有挑战性。此前,我们从 Gluconobacter oxydans DSM2343 中发现了一种醇脱氢酶 Gox0525,它具有严格的非对映选择性(d.e. 值为 99%),但对 Fop 二酮的活性较低。为了提高 Gox0525 的催化效率,本研究在定点饱和诱变的基础上进行了基于聚焦理性迭代位点特异性诱变(FRISM)的半理性设计。与野生型 Gox0525 相比,变体 M4(Y92G/P93M/Y94P/L151V)的催化效率(Kcat/Km)提高了 64 倍,比活性提高了 47 倍。将共同表达变体 M4 和用于 NADPH 再生的枯草芽孢杆菌葡萄糖脱氢酶(BsGDH)的工程大肠杆菌细胞用作生物催化剂,进行克级规模的 Fop 二酮反应。结果,95 mM (33.76 g/L) Fop dione 在 4 h 内被完全转化,得到(S)-Fop 醇,d.e. 值为 99%,产率为 96%,时空产率为 195.6 g/L/d。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


