Exploring the factors influencing the ketoenamine-enolimine tautomeric equilibrium of pyridoxal 5′-phosphate in branched-chain aminotransferases
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Pyridoxal 5′-phosphate (PLP), the active form of vitamin B6, is a critical coenzyme for various enzymes. It generates a Schiff base with the substrate and exhibits ketoenamine and enolimine tautomeric forms due to intramolecular proton transfer. This study aims to ascertain the predominant tautomeric form of the PLP Schiff base in MtIlvE and analyze its influencing factors. Molecular dynamics simulations indicate that the ketoenamine tautomer has higher binding free energy than the enolimine tautomer. Density functional theory calculations suggest that, despite their ability to interconvert at a relatively low energy barrier, the ketoenamine tautomer is thermodynamically more stable. Factors affecting the keto-enol tautomeric equilibrium were investigated by constructing various QM-cluster models. Our results demonstrate that both the protonation of the pyridine nitrogen and the presence of Tyr209, which stabilizes the O3 anion, shift the tautomeric equilibrium toward the ketoenamine configuration. These findings provide a theoretical basis for investigating enzyme catalytic mechanisms.
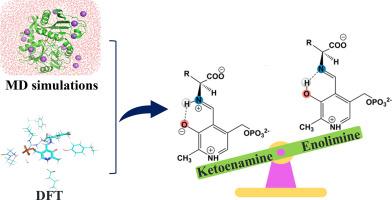
支链氨基转移酶中影响 5′-磷酸吡哆醛酮胺-烯醇亚胺同分异构平衡的因素探究
吡哆醛 5′-磷酸(PLP)是维生素 B6 的活性形式,是各种酶的重要辅酶。它与底物生成希夫碱,并由于分子内质子转移而呈现酮烯胺和烯醇亚胺同分异构体形式。本研究旨在确定 MtIlvE 中 PLP 席夫碱的主要同分异构体形式,并分析其影响因素。分子动力学模拟表明,酮烯胺同分异构体的结合自由能高于烯丙基亚胺同分异构体。密度泛函理论计算表明,尽管酮烯胺同系物能够在相对较低的能障下相互转化,但其热力学稳定性更高。通过构建各种 QM-簇模型,研究了影响酮烯醇同分异构体平衡的因素。我们的研究结果表明,吡啶氮的质子化和 Tyr209 的存在都会使同分异构体的平衡向酮烯胺构型移动,而 Tyr209 能稳定 O3 阴离子。这些发现为研究酶催化机理提供了理论基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


