Design and synthesis of dicyanoethylene derivatives functionalized with carbazole possessing the properties of obvious aggregation-induced emission and reversible high-contrast mechanofluorochromism
IF 3.3
3区 物理与天体物理
Q2 OPTICS
引用次数: 0
Abstract
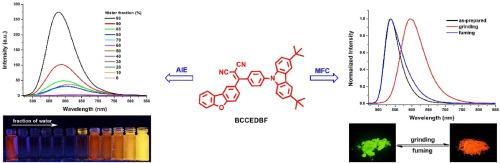
This study devotes to the design, preparation, and examination of two novel D-A structured molecules, designated as CCEDBF and BCCEDBF, which are derived from carbazole unit, dicyanoethylene segment, and dibenzofuran unit. The distinctive D-A molecular architectures and highly twisted spatial configurations of these compounds facilitate pronounced intramolecular charge transfer (ICT) while also imparting robust solid-state luminescence, exhibiting emission efficiencies of 0.518 and 0.739, respectively, and notable aggregation-induced emission (AIE) with AIE factors of 43 and 30. Significantly, both CCEDBF and BCCEDBF demonstrate reversible mechanofluorochromic (MFC) behavior characterized by high fluorescence contrast. Mechanical grinding of the initial powders results in a shift in their emission colors, transitioning from green and yellow-green to yellow and orange-red, respectively, alongside a corresponding shift in emission peaks from 525 nm to 536 nm–558 nm and 597 nm. Powder X-ray diffraction (PXRD) examination of the initially synthesized, mechanically processed, and vaporized samples reveals that the observed fluorescence color change is due to a structural transformation between ordered crystalline and disordered amorphous configurations induced by the application of the external force. The red shift in photoluminescence (PL) spectra post-grinding is attributed to a reduced band gap, driven by factors such as extended π-conjugation length, enhanced planar intramolecular charge transfer (PICT) effect, strengthened π-π interactions and exciton coupling, as well as the increase in the orbitals overlap between neighboring molecular. Moreover, the presence or absence of tert-butyl substituents attaching to the 3- and 6-positions of the carbazole units significantly impacts the photophysical properties of these materials.
设计和合成具有明显聚集诱导发射和可逆高对比度机械荧光特性的咔唑官能化二氰基乙烯衍生物
本研究致力于设计、制备和检验两种新型 D-A 结构分子,分别命名为 CCEDBF 和 BCCEDBF,它们由咔唑单元、二氰基乙烯段和二苯并呋喃单元衍生而来。这些化合物独特的 D-A 型分子结构和高度扭曲的空间构型促进了明显的分子内电荷转移(ICT),同时也赋予了它们强大的固态发光能力,其发射效率分别为 0.518 和 0.739,聚集诱导发射(AIE)效果显著,AIE 系数分别为 43 和 30。值得注意的是,CCEDBF 和 BCCEDBF 都表现出以高荧光对比度为特征的可逆机械荧光变色(MFC)行为。对初始粉末进行机械研磨会导致它们的发射颜色发生变化,分别从绿色和黄绿色过渡到黄色和橙红色,同时发射峰也相应地从 525 纳米变为 536 纳米-558 纳米和 597 纳米。对初步合成、机械加工和气化的样品进行的粉末 X 射线衍射(PXRD)检查表明,观察到的荧光颜色变化是由于外力作用引起的有序结晶和无序非晶构型之间的结构转变。研磨后光致发光(PL)光谱的红移归因于带隙的减小,其驱动因素包括π-共轭长度的延长、平面分子内电荷转移(PICT)效应的增强、π-π相互作用和激子耦合的加强以及相邻分子间轨道重叠的增加。此外,咔唑单元的 3 位和 6 位是否存在叔丁基取代基对这些材料的光物理性质也有显著影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Luminescence
物理-光学
CiteScore
6.70
自引率
13.90%
发文量
850
审稿时长
3.8 months
期刊介绍:
The purpose of the Journal of Luminescence is to provide a means of communication between scientists in different disciplines who share a common interest in the electronic excited states of molecular, ionic and covalent systems, whether crystalline, amorphous, or liquid.
We invite original papers and reviews on such subjects as: exciton and polariton dynamics, dynamics of localized excited states, energy and charge transport in ordered and disordered systems, radiative and non-radiative recombination, relaxation processes, vibronic interactions in electronic excited states, photochemistry in condensed systems, excited state resonance, double resonance, spin dynamics, selective excitation spectroscopy, hole burning, coherent processes in excited states, (e.g. coherent optical transients, photon echoes, transient gratings), multiphoton processes, optical bistability, photochromism, and new techniques for the study of excited states. This list is not intended to be exhaustive. Papers in the traditional areas of optical spectroscopy (absorption, MCD, luminescence, Raman scattering) are welcome. Papers on applications (phosphors, scintillators, electro- and cathodo-luminescence, radiography, bioimaging, solar energy, energy conversion, etc.) are also welcome if they present results of scientific, rather than only technological interest. However, papers containing purely theoretical results, not related to phenomena in the excited states, as well as papers using luminescence spectroscopy to perform routine analytical chemistry or biochemistry procedures, are outside the scope of the journal. Some exceptions will be possible at the discretion of the editors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


