One arrow two eagles: Multifunctional nano-system for macrophage reprogramming and osteoclastogenesis inhibition against inflammatory osteolysis
IF 8.7
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Inflammatory osteolysis poses a significant worldwide threat to public health. However, current monotherapies, which target either the prevention of the inflammatory response or the attenuation of osteoclast (OC) formation, have limited efficacy due to the complexity of the bone immune system being overlooked. Herein, by means of modifying salmon calcitonin (sCT), a multifunctional nano-system (AuNDs-sCT) was designed to synergistically inhibit OC differentiation and reverse the inflammatory microenvironment against inflammatory osteolysis. On the one hand, AuNDs-sCT effectively restrained OC differentiation by binding to the calcitonin receptors on the surface of OC precursors, resulting in the down-regulation of OC-specific genes and proteins. The targeted capacity of AuNDs-sCT provided a more durable and precise therapeutic effect. On the other hand, AuNDs-sCT exhibited antioxidant and anti-inflammatory effects, which regulated the polarization “switch” from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype in macrophages by the inhibition of NF-κB p65 phosphorylation, thereby effectively reversed the local inflammatory microenvironment. Additionally, AuNDs-sCT served as a promising fluorescent probe, enabling real-time visualization of the therapeutic process. This capability is expected to optimize drug administration and evaluate therapeutic effects. In summary, by inhibiting OC differentiation and reprogramming macrophages, AuNDs-sCT successfully realized drug repurposing and achieved the “one arrow two eagles” therapeutic strategy, which offers a synergistic and effective treatment option for the clinical management of inflammatory osteolysis.
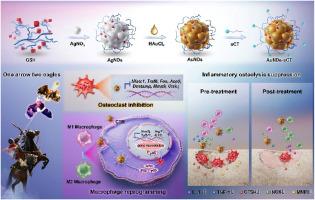
一箭双雕用于巨噬细胞重编程和抑制破骨细胞生成以对抗炎症性骨溶解的多功能纳米系统
炎症性骨溶解对全球公众健康构成重大威胁。然而,由于忽视了骨免疫系统的复杂性,目前针对预防炎症反应或抑制破骨细胞(OC)形成的单一疗法疗效有限。本文通过对鲑鱼降钙素(sCT)进行修饰,设计出一种多功能纳米系统(AuNDs-sCT),可协同抑制破骨细胞分化,逆转炎症微环境,防止炎症性骨溶解。一方面,AuNDs-sCT通过与OC前体表面的降钙素受体结合,有效抑制OC分化,从而导致OC特异性基因和蛋白的下调。AuNDs-sCT 的靶向能力提供了更持久、更精确的治疗效果。另一方面,AuNDs-sCT 具有抗氧化和抗炎作用,通过抑制 NF-κB p65 磷酸化,调节巨噬细胞从促炎 M1 表型向抗炎 M2 表型的极化 "切换",从而有效逆转局部炎症微环境。此外,AuNDs-sCT 还可作为一种前景看好的荧光探针,实现治疗过程的实时可视化。这一功能有望优化给药和评估治疗效果。总之,AuNDs-sCT通过抑制OC分化和重编程巨噬细胞,成功实现了药物的再利用,实现了 "一箭双雕 "的治疗策略,为临床治疗炎性骨溶解提供了一种协同有效的治疗方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


