Simultaneous targeting and suppression of heat shock protein 60 to overcome heat resistance and induce mitochondrial death of tumor cells in photothermal immunotherapy
IF 8.7
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
As the most aggressive and metastatic subtype of breast cancer, clinical demands of triple negative breast cancer (TNBC) have far not been met. Heat shock protein 60 (HSP60) is over expressed in tumor cells and impair the efficacy of photothermal therapy. In this work, a conjugate composed of self-designed peptide targeting HSP60 and gold nanorods was constructed, referred to as AuNR-P17. Results showed that AuNR-P17 was able to simultaneously down regulate the level of HSP60 and locate in the mitochondria where HSP60 is enriched in the tumor cells of TNBC, which also impeded the interaction between HSP60 and integrin α3, thereby reducing the tumor cells' heat tolerance and metastatic capabilities. At the same time, AuNR-P17 induced remarkable mitochondrial apoptosis when exposed to the laser irradiation of 808 nm. The dual functions of AuNR-P17 led to the decrement of BCL-2 and the activation of p53 and cleaved caspase-3. The danger associated molecular patterns (DAMPs) generated from the mitochondrial apoptosis elicited strong and long-term specific immune responses against TNBC in vivo and ultimately inhibited the tumor metastasis and recurrence with significantly prolonged survival (>100 days) on TNBC mice. In conclusion, this study demonstrated HSP60 a promising potential therapeutic target for triple negative breast cancer and exhibited powerful capacity of AuNR-P17 in photothermal immune therapy.
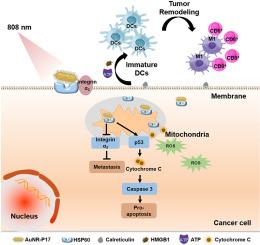
在光热免疫疗法中同时靶向和抑制热休克蛋白 60 以克服耐热性并诱导肿瘤细胞线粒体死亡
作为乳腺癌中最具侵袭性和转移性的亚型,三阴性乳腺癌(TNBC)的临床需求远未得到满足。热休克蛋白60(HSP60)在肿瘤细胞中过度表达,会影响光热疗法的疗效。本研究构建了一种由自主设计的靶向 HSP60 的多肽和金纳米棒组成的共轭物,称为 AuNR-P17。结果表明,AuNR-P17能同时下调HSP60的水平,并定位在TNBC肿瘤细胞中HSP60富集的线粒体中,还能阻碍HSP60与整合素α3的相互作用,从而降低肿瘤细胞的耐热性和转移能力。同时,AuNR-P17 在 808 纳米激光照射下可诱导线粒体显著凋亡。AuNR-P17 的双重功能导致了 BCL-2 的降低、p53 和裂解的 caspase-3 的激活。线粒体凋亡产生的危险相关分子模式(DAMPs)在体内引起了针对 TNBC 的强烈而长期的特异性免疫反应,并最终抑制了肿瘤的转移和复发,显著延长了 TNBC 小鼠的生存期(100 天)。总之,这项研究证明了HSP60是治疗三阴性乳腺癌的潜在靶点,并展示了AuNR-P17在光热免疫疗法中的强大能力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


