Biomimetic composite gelatin methacryloyl hydrogels for improving survival and osteogenesis of human adipose-derived stem cells in 3D microenvironment
IF 8.7
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
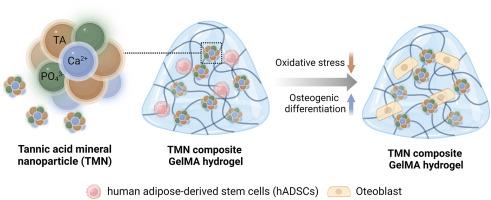
Gelatin methacryloyl (GelMA) hydrogels are used for stem cell encapsulation in bone tissue engineering due to their fast and stable photo-crosslinking. However, cell viability and ability to induce osteogenesis are reduced by reactive oxygen species (ROS) produced during the crosslinking reaction. In this study, we developed biomimetic nanoparticles (TMNs) by combining tannic acid (TA) and simulated body fluid (SBF) minerals, and used them to synthesize GelMA-based composite hydrogels for addressing those limitations. The optimal concentrations of TA and SBF were investigated to create nanoparticles that can effectively scavenge ROS and induce osteogenesis. The incorporation of TMNs into composite hydrogels (G-TMN) significantly enhanced the survival and proliferation of encapsulated human adipose-derived stem cells (hADSCs) by providing resistance to oxidative conditions. In addition, the ions that were released, such as Ca2+ and PO43−, stimulated stem cell differentiation into bone cells. The hADSCs encapsulated in G-TMN had 2.0 ± 0.8-fold greater viability and 1.3 ± 1.8 times greater calcium deposition than those encapsulated in the hydrogel without nanoparticles. Furthermore, the in vivo transplantation of G-TMN into a subcutaneous mouse model demonstrated the rapid degradation of the gel-network while retaining the osteoinductive particles and cells in the transplanted area. The increased cellular activity observed in our multifunctional composite hydrogel can serve as a foundation for novel and effective therapies for bone deformities.
仿生复合明胶甲基丙烯酰水凝胶用于提高人脂肪来源干细胞在三维微环境中的存活率和成骨率
明胶甲基丙烯酰(GelMA)水凝胶因其快速稳定的光交联作用而被用于骨组织工程中的干细胞封装。然而,交联反应过程中产生的活性氧(ROS)会降低细胞活力和诱导骨生成的能力。在这项研究中,我们结合单宁酸(TA)和模拟体液(SBF)矿物质开发了仿生物纳米颗粒(TMNs),并用它们合成了基于 GelMA 的复合水凝胶,以解决这些局限性。研究了TA和SBF的最佳浓度,以制造出能有效清除ROS并诱导成骨的纳米颗粒。在复合水凝胶(G-TMN)中加入 TMN,可通过提供抗氧化能力显著提高包裹的人脂肪来源干细胞(hADSCs)的存活和增殖。此外,释放的离子(如 Ca2+ 和 PO43-)可刺激干细胞分化为骨细胞。与不含纳米颗粒的水凝胶相比,封装在G-TMN中的hADSCs活力高出2.0 ± 0.8倍,钙沉积量高出1.3 ± 1.8倍。此外,将 G-TMN 移植到小鼠皮下模型的体内试验表明,凝胶网络降解迅速,但骨诱导颗粒和细胞仍保留在移植区域。在我们的多功能复合水凝胶中观察到的细胞活性的提高,可以为新型、有效的骨畸形疗法奠定基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


