Elucidating the gas cell stabilization mechanism of buckwheat-wheat steamed bread induced by transglutaminase: A focus on the foaming and air-water interfacial properties of dough liquor
IF 11
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
This work investigated the improvement mechanism of transglutaminase (TG) on the buckwheat-wheat steamed bread qualities from the view of protein structural, foaming and air-water interfacial properties of dough liquor (DL). TG reduced the hardness, ameliorated the crumb structure and enhanced the specific volume of buckwheat-wheat steamed bread by up to 9.0%. The DL yield, protein and water recovery were notably increased as the TG concentration increased to 0.2 U/g, then decreased with the higher TG concentrations. The low TG concentrations (<0.2 U/g) increased the protein molecular weight, and decreased the protein hydrophobicity, free amino and sulfhydryl groups content of DL. The high TG concentrations (>0.2 U/g) tended to induce both protein intermolecular and intramolecular cross-linking, which reduced the average particle size from 9.6 to 4.3 μm as the TG concentration increased from 0.2 to 1.0 U/g. TG markedly improved the foaming capacity and foam stability, decreased the surface tension and strengthened the interfacial films by enhancing the viscoelasticity modulus of buckwheat-wheat DL interfacial protein films. The topography of the air-water interfacial layer revealed that the TG-induced protein cross-linking led to a more homogeneous and denser topographical structure. This work will provide fresh insight into the effects of TG-induced protein cross-linking on gas cell stabilization and relate them to the buckwheat-wheat steamed bread quality properties.
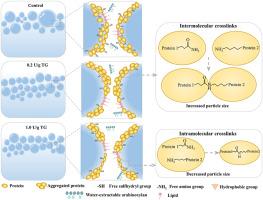
阐明转谷氨酰胺酶诱导的荞麦-小麦馒头气孔稳定机制关注面团液的发泡和气水界面特性
本研究从面团液(DL)的蛋白质结构、发泡和气水界面特性的角度研究了转谷氨酰胺酶(TG)对荞麦-小麦馒头品质的改善机理。TG 降低了荞麦-小麦馒头的硬度,改善了面包屑结构,并使其比容提高了 9.0%。当 TG 浓度增加到 0.2 U/g 时,DL 产量、蛋白质和水分回收率显著增加,然后随着 TG 浓度的增加而降低。低TG浓度(0.2 U/g)增加了DL的蛋白质分子量,降低了蛋白质的疏水性、游离氨基和巯基含量。高浓度(0.2 U/g)的 TG 有诱导蛋白质分子间和分子内交联的趋势,随着 TG 浓度从 0.2 U/g增加到 1.0 U/g,平均粒径从 9.6 μm 减小到 4.3 μm。TG 显著提高了发泡能力和泡沫稳定性,降低了表面张力,并通过提高荞麦-小麦 DL 界面蛋白膜的粘弹性模量来增强界面膜。空气-水界面层的形貌显示,TG 诱导的蛋白质交联产生了更均匀、更致密的形貌结构。这项研究将有助于深入了解 TG 诱导的蛋白质交联对气孔稳定的影响,并将其与荞麦-小麦馒头的质量特性联系起来。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Hydrocolloids
工程技术-食品科技
CiteScore
19.90
自引率
14.00%
发文量
871
审稿时长
37 days
期刊介绍:
Food Hydrocolloids publishes original and innovative research focused on the characterization, functional properties, and applications of hydrocolloid materials used in food products. These hydrocolloids, defined as polysaccharides and proteins of commercial importance, are added to control aspects such as texture, stability, rheology, and sensory properties. The research's primary emphasis should be on the hydrocolloids themselves, with thorough descriptions of their source, nature, and physicochemical characteristics. Manuscripts are expected to clearly outline specific aims and objectives, include a fundamental discussion of research findings at the molecular level, and address the significance of the results. Studies on hydrocolloids in complex formulations should concentrate on their overall properties and mechanisms of action, while simple formulation development studies may not be considered for publication.
The main areas of interest are:
-Chemical and physicochemical characterisation
Thermal properties including glass transitions and conformational changes-
Rheological properties including viscosity, viscoelastic properties and gelation behaviour-
The influence on organoleptic properties-
Interfacial properties including stabilisation of dispersions, emulsions and foams-
Film forming properties with application to edible films and active packaging-
Encapsulation and controlled release of active compounds-
The influence on health including their role as dietary fibre-
Manipulation of hydrocolloid structure and functionality through chemical, biochemical and physical processes-
New hydrocolloids and hydrocolloid sources of commercial potential.
The Journal also publishes Review articles that provide an overview of the latest developments in topics of specific interest to researchers in this field of activity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


